SEARCH RESULTS FOR: heart failure
Left Heart Failure - Pathogenesis
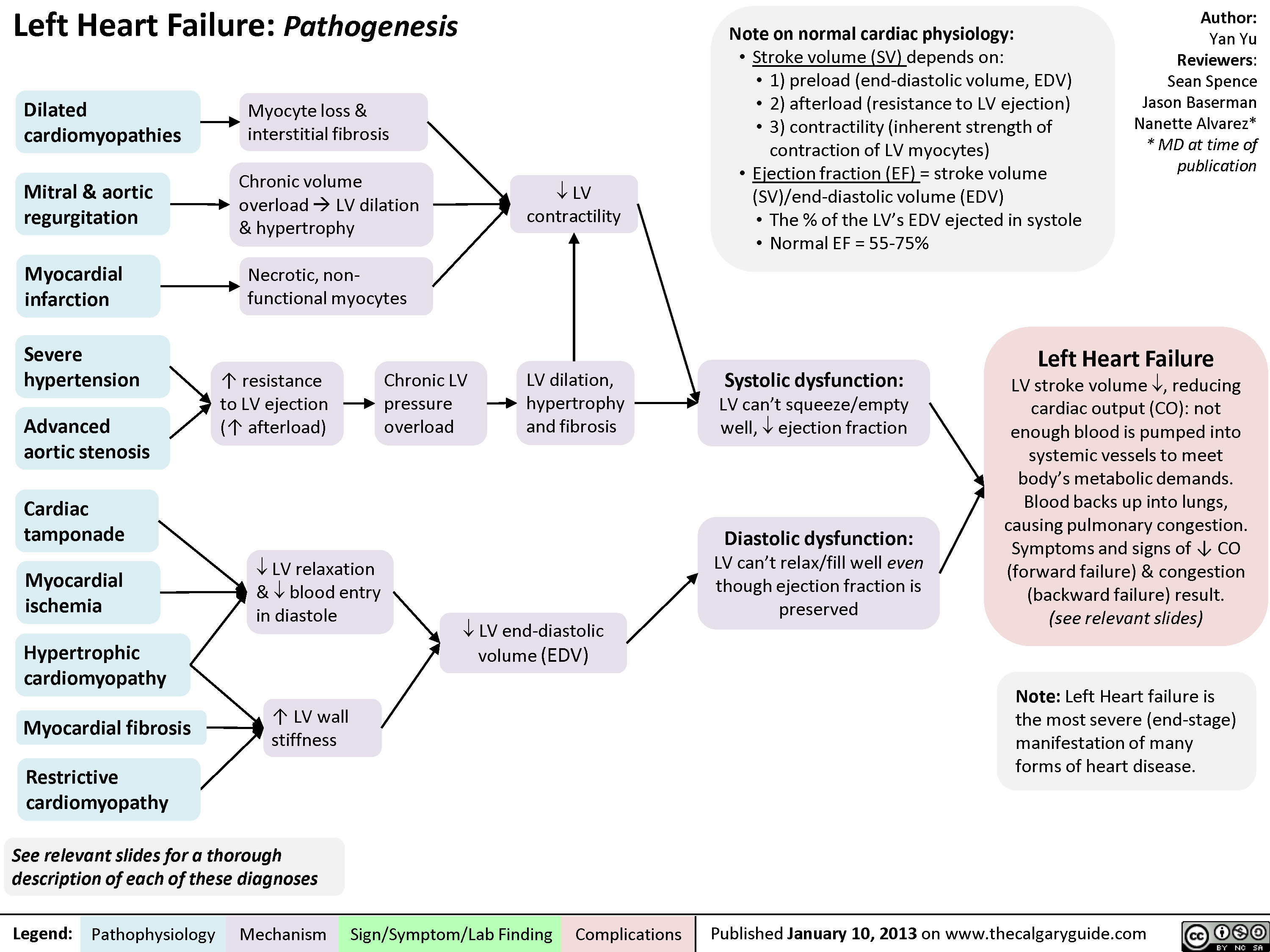
Right Heart Failure
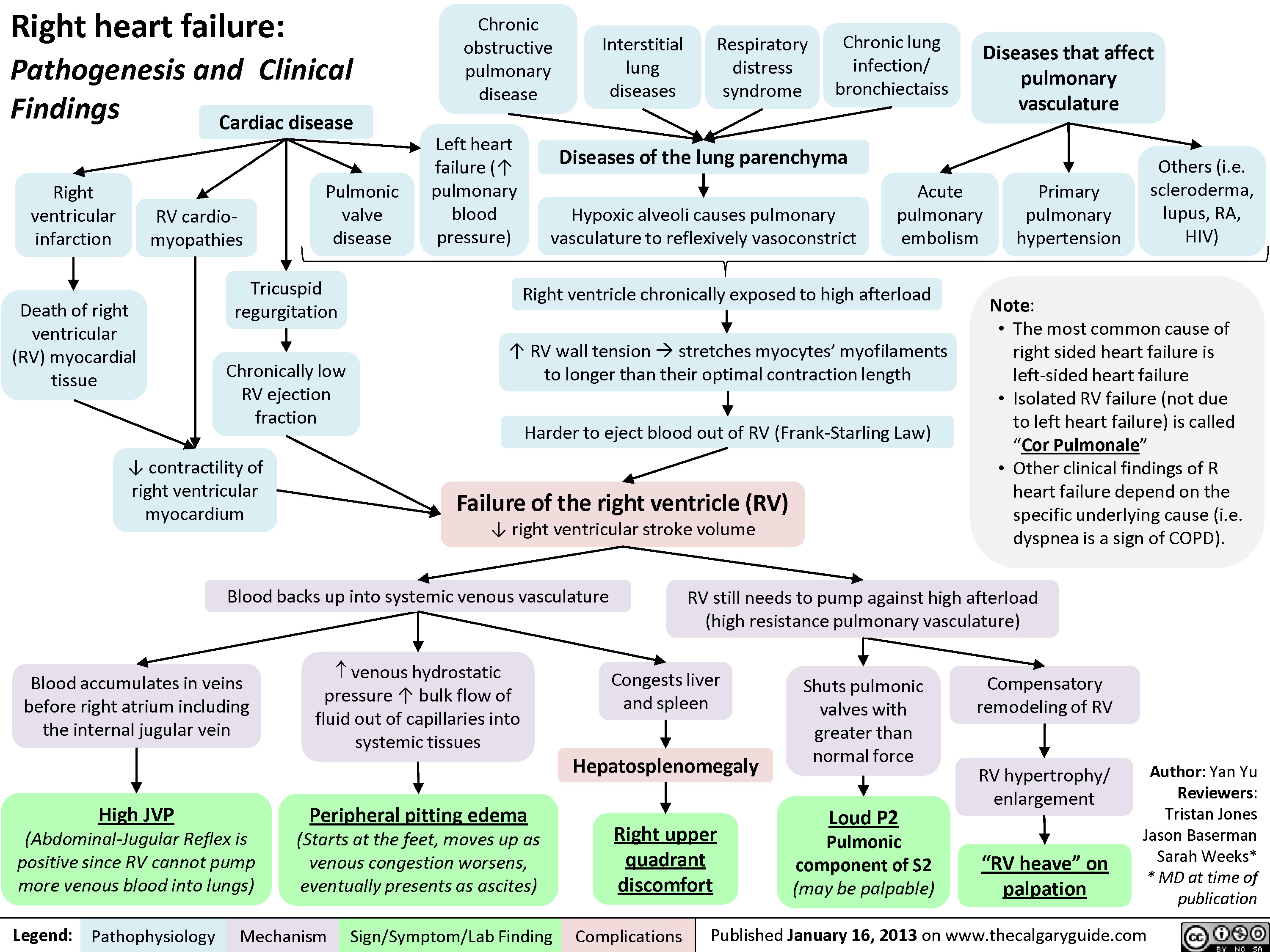
Left Heart Failure
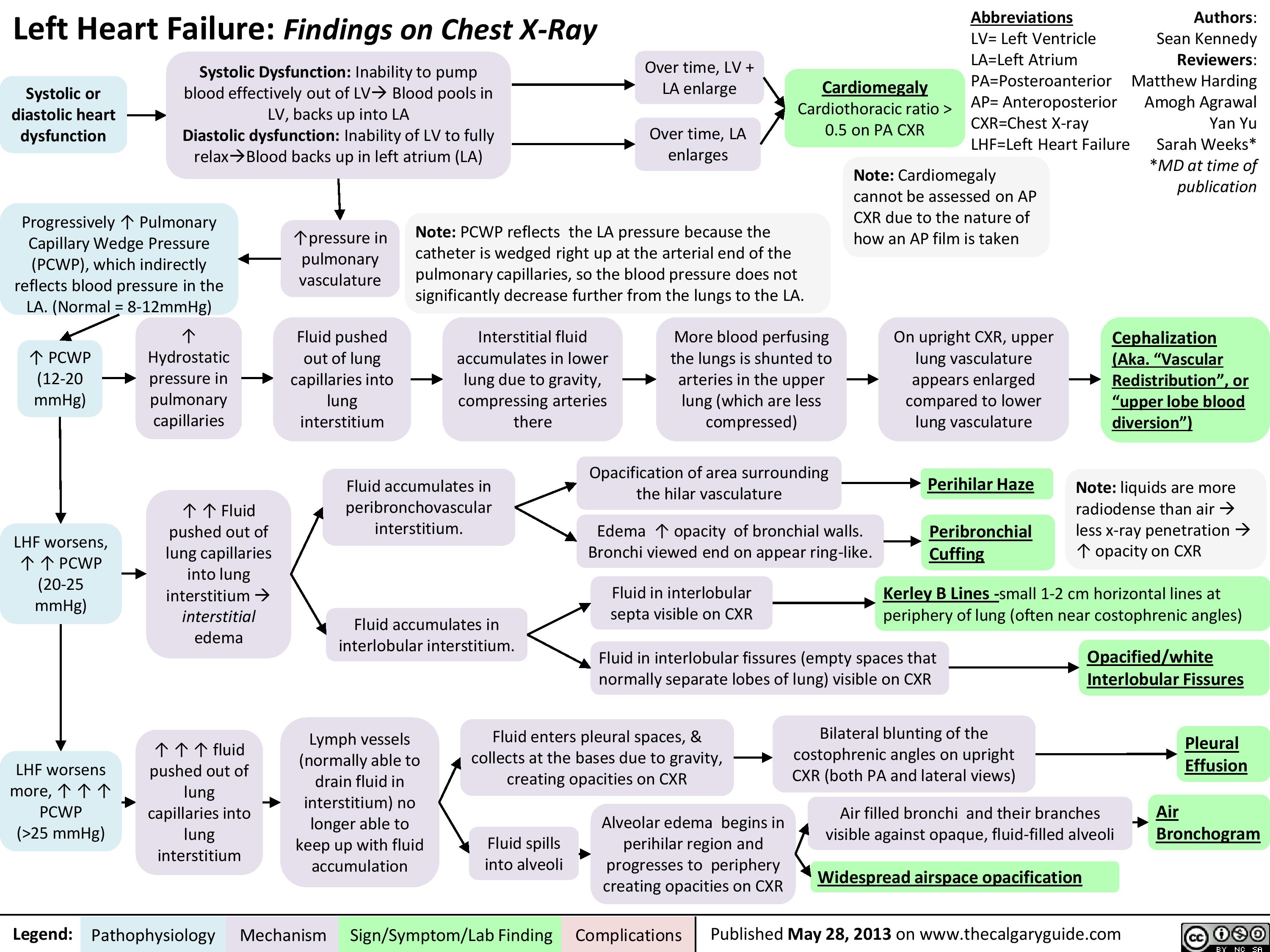
Left Heart Failure - Physical Exam Findings
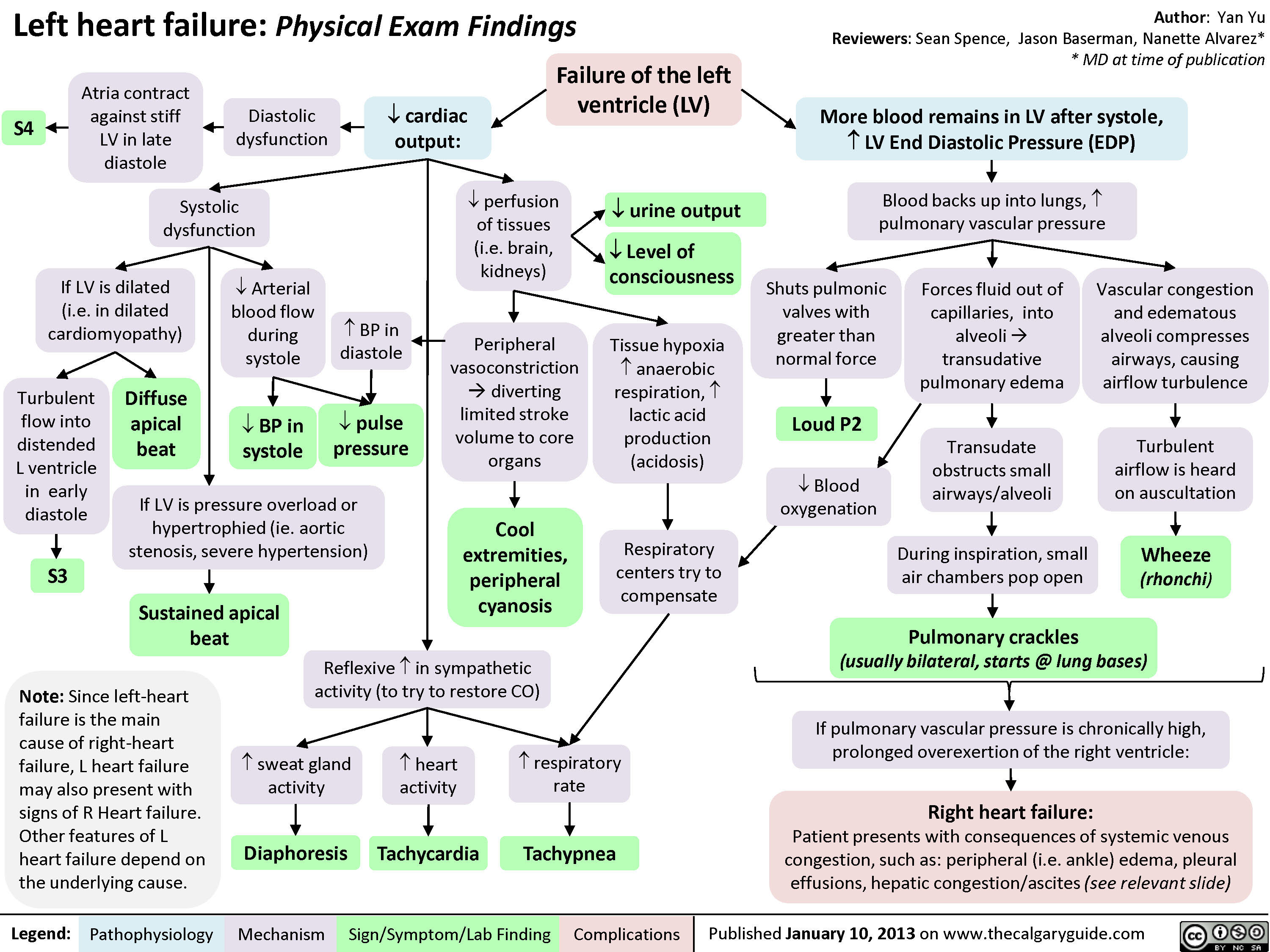
Left Heart Failure - Findings on History
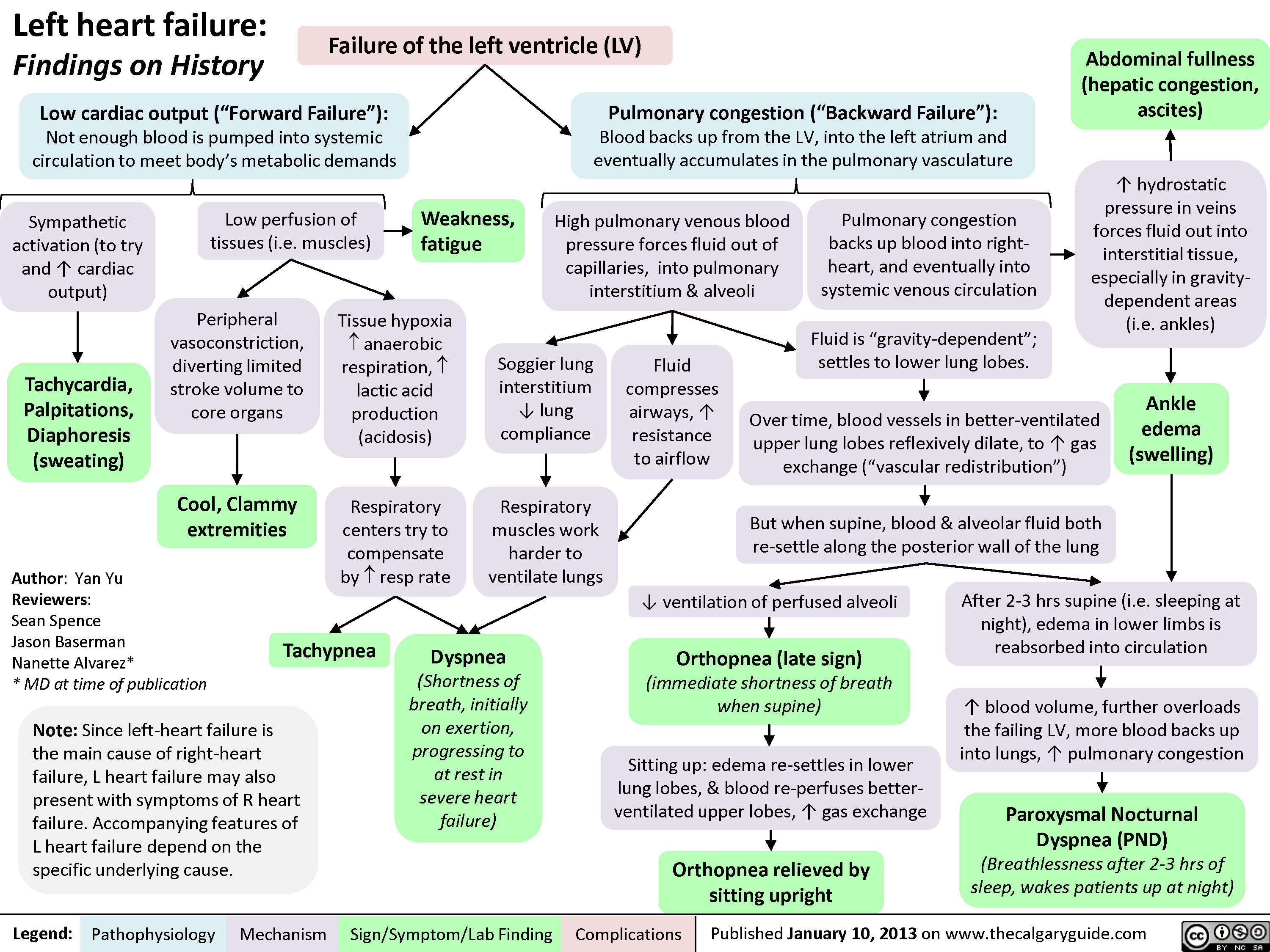
Left Heart Failure: Pathophysiology (Neurohormonal Activation)
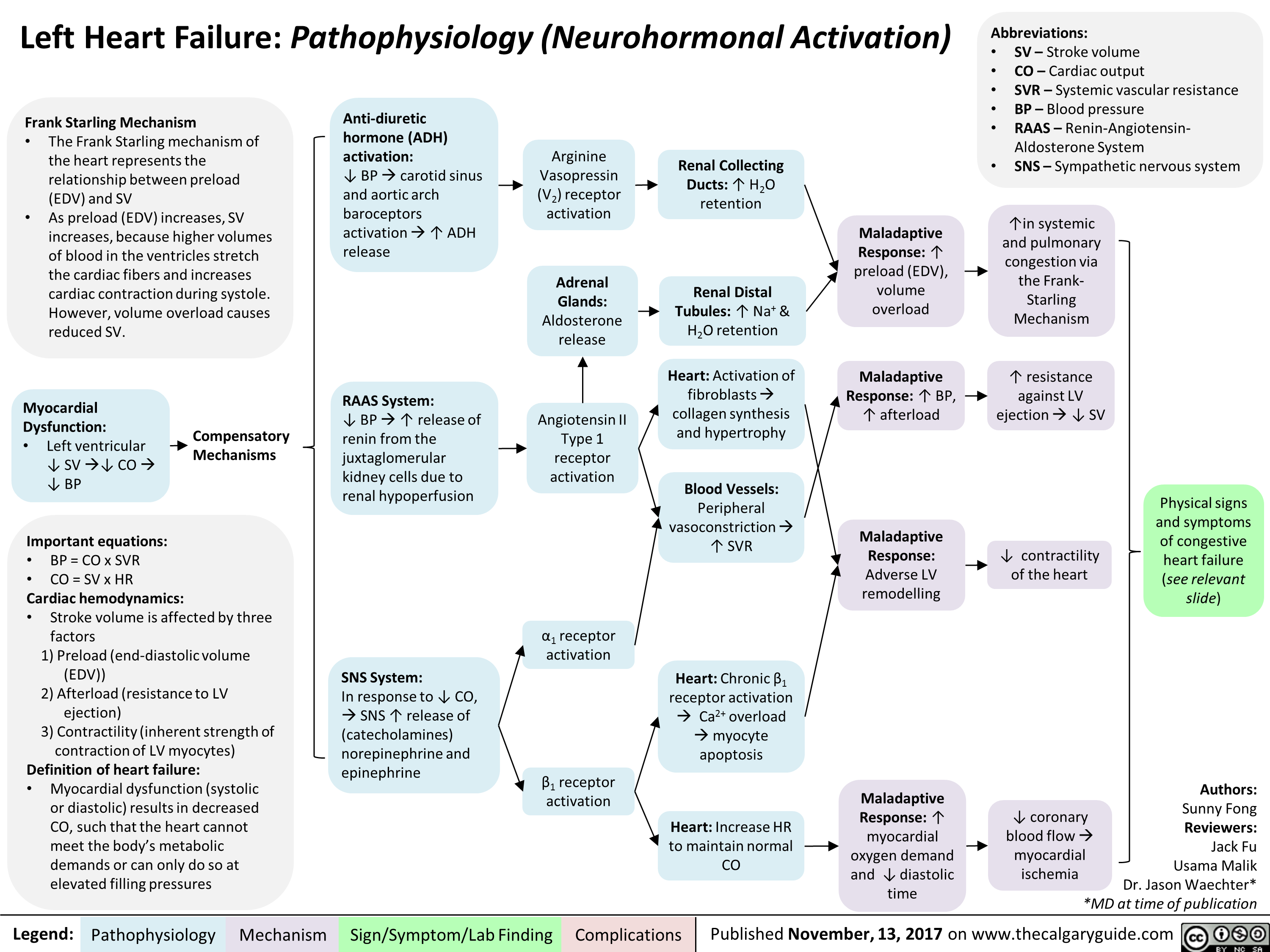
Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Pathogenesis
Hyponatremia- Physiology
![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)
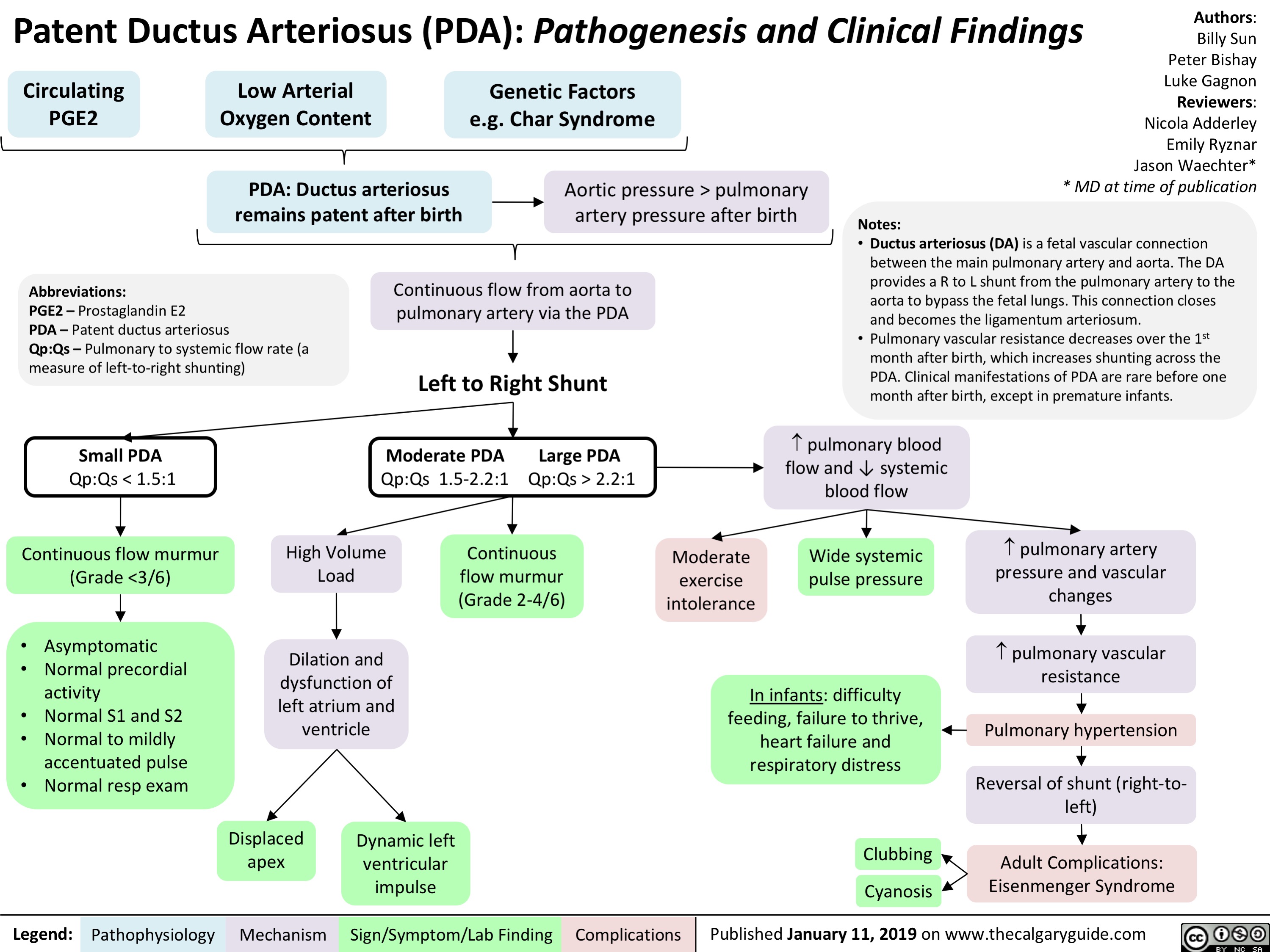
Patent Ductus Arteriosus (PDA)- Pathogenesis and Clinical Findings
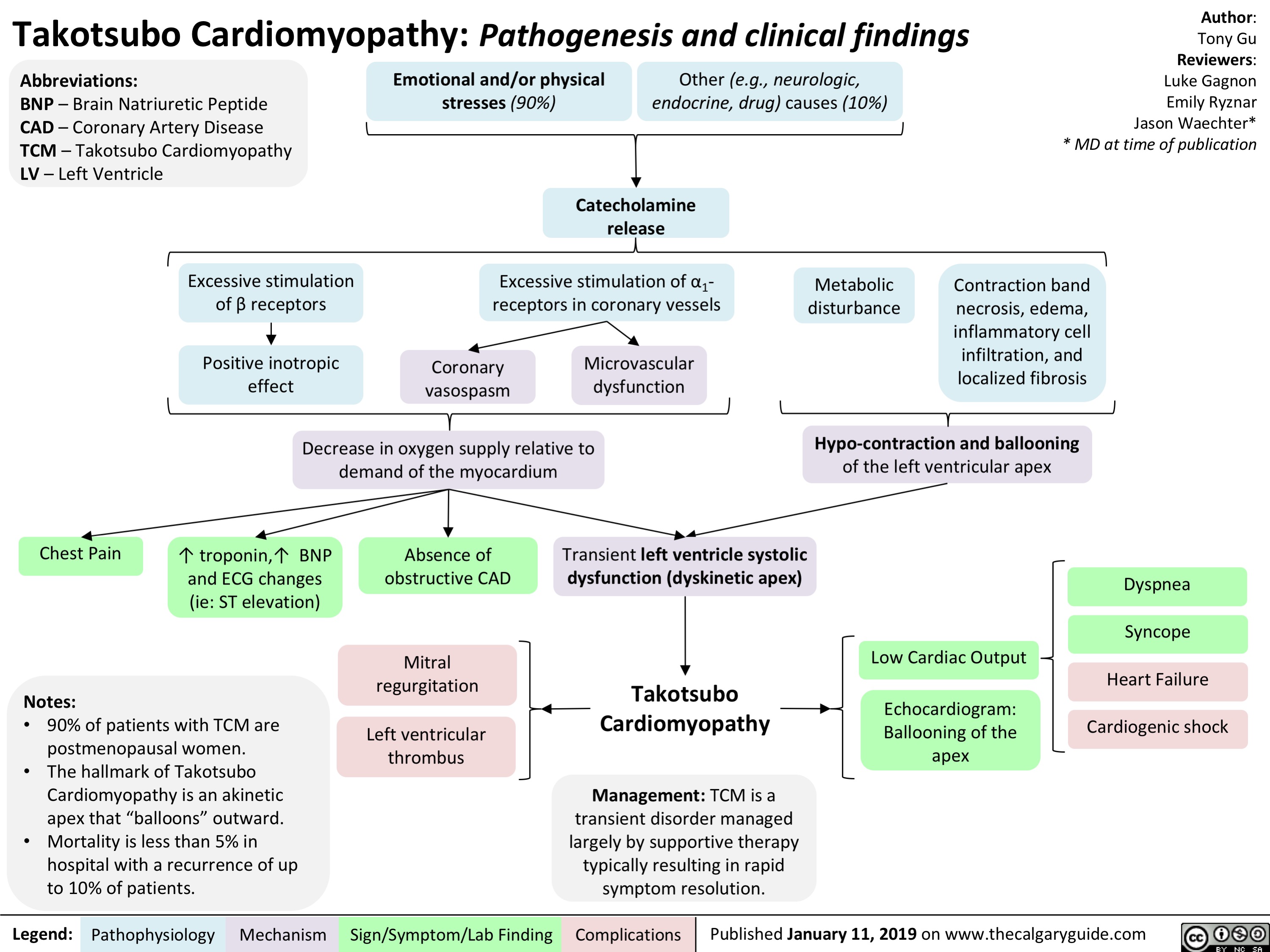
Takotsubo Cardiomyopathy- Pathogenesis and clinical findings
Hyperkalemia- Physiology
![Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hyperkalemia-Physiology-.jpg)
Torsades de Pointes (TdP)- Pathogenesis and Clinical Findings
![Torsades de Pointes (TdP): Pathogenesis and Clinical Findings
Drugs (e.g. Class 1A [quinidine], Class III [sotalol,
amiodarone], TCAs, erythromycin, quinolones, anti-histamines)
Sinus bradycardia, AV block
Metabolic abnormality (hypo K+/Ca2+/Mg2+)
Primary heart disease: ischemic, congestive heart failure, cardiomyopathy
Acquired long QT syndrome
Congenital long QT syndrome
↓ repolarizing current/ depolarizing current in cardiomyocytes
Mutated cardiac ion channels
Author: Nicola Adderley Reviewers: Luke Gagnon Emily Ryznar *Saman Rezazadeh *George Veenhuyzen MD at time of publication*
↓ repolarizing current in cardiomyocytes
QTc interval
Prolonged ventricular action potential duration
Early after depolarization (EAD) triggering PVC
Torsades de Pointes
Illustrated changes to action potential:
Normal cardiac action potential
EAD
Abbreviations: TCAs: tricyclic antidepressants AV: atrioventricular QTc: QT interval, corrected for heart rate
PVC: premature ventricular contraction
VF: ventricular fibrillation
SR: sinus rhythm
Polymorphic ventricular tachycardia initiated by PVC in the setting of QT interval prolongation and maintained by functional re-entry
Non-sustained TdP
Asymptomatic, palpitations, syncope (length-dependent)
Illustrated changes to ECG Strip:
OR
Sinus rhythm restored
Degeneration to VF
Sudden cardiac death
Prolonged repolarization
Triggered beat (PVC)
A
BCDE
A. Prolonged QT interval B. PVC
C. PVC triggers TdP
D. Non-sustained TdP
E. Return to SR
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 10, 2019 on www.thecalgaryguide.com
Torsades de Pointes (TdP): Pathogenesis and Clinical Findings
Drugs (e.g. Class 1A [quinidine], Class III [sotalol,
amiodarone], TCAs, erythromycin, quinolones, anti-histamines)
Sinus bradycardia, AV block
Metabolic abnormality (hypo K+/Ca2+/Mg2+)
Primary heart disease: ischemic, congestive heart failure, cardiomyopathy
Acquired long QT syndrome
Congenital long QT syndrome
↓ repolarizing current/ depolarizing current in cardiomyocytes
Mutated cardiac ion channels
Author: Nicola Adderley Reviewers: Luke Gagnon Emily Ryznar *Saman Rezazadeh *George Veenhuyzen MD at time of publication*
↓ repolarizing current in cardiomyocytes
QTc interval
Prolonged ventricular action potential duration
Early after depolarization (EAD) triggering PVC
Torsades de Pointes
Illustrated changes to action potential:
Normal cardiac action potential
EAD
Abbreviations: TCAs: tricyclic antidepressants AV: atrioventricular QTc: QT interval, corrected for heart rate
PVC: premature ventricular contraction
VF: ventricular fibrillation
SR: sinus rhythm
Polymorphic ventricular tachycardia initiated by PVC in the setting of QT interval prolongation and maintained by functional re-entry
Non-sustained TdP
Asymptomatic, palpitations, syncope (length-dependent)
Illustrated changes to ECG Strip:
OR
Sinus rhythm restored
Degeneration to VF
Sudden cardiac death
Prolonged repolarization
Triggered beat (PVC)
A
BCDE
A. Prolonged QT interval B. PVC
C. PVC triggers TdP
D. Non-sustained TdP
E. Return to SR
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 10, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Torsades-de-Pointes-TdP-Pathogenesis-and-Clinical-Findings-.jpg)
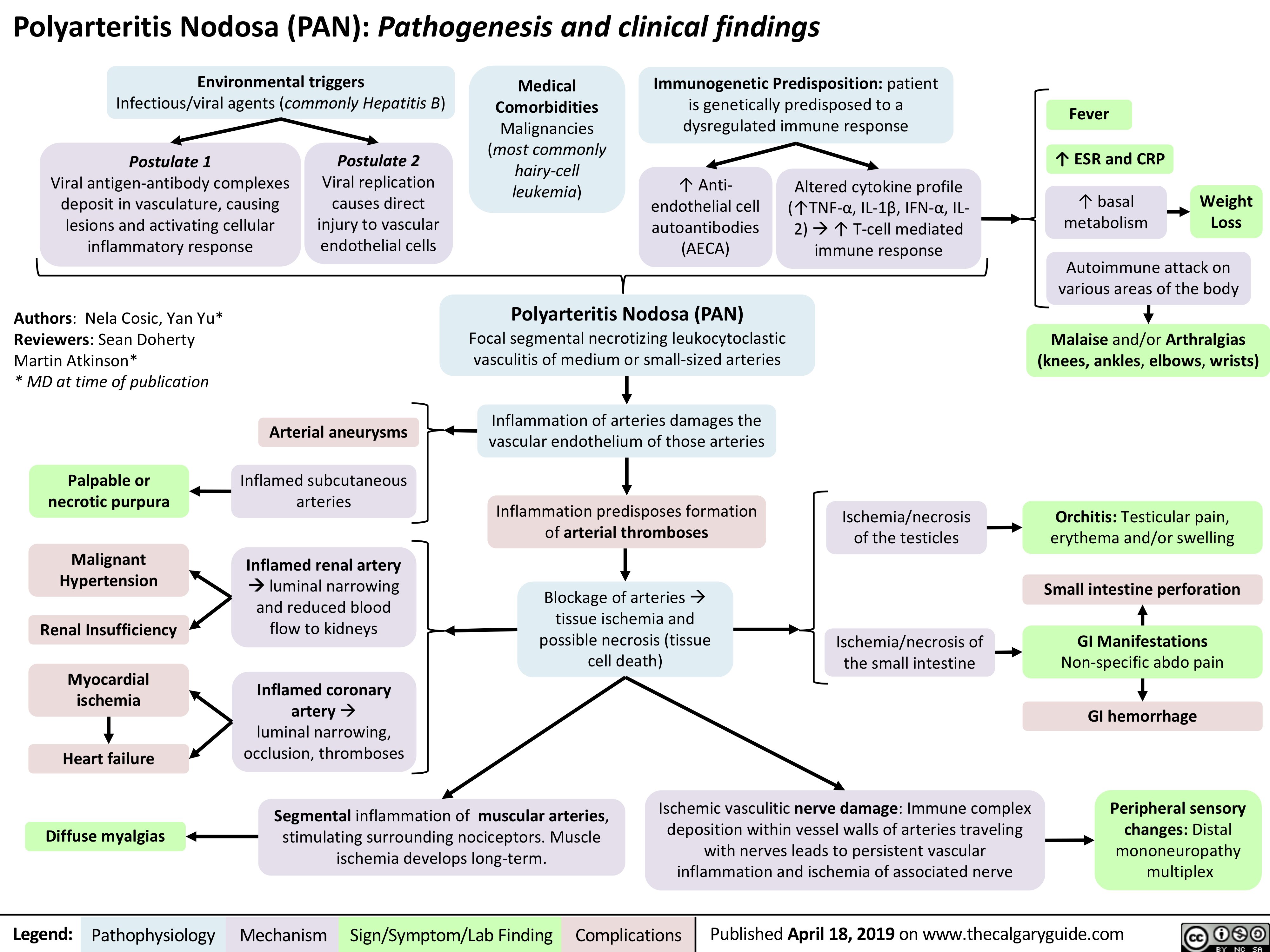
Polyarteritis Nodosa (PAN): Pathogenesis and Clinical Findings
Normocytic Anemia
![Normocytic Anemia: Causes, Signs, and Symptoms
Authors: Katie Lin Yan Yu Reviewers: Andrew Brack Jessica Tjong Man-Chiu Poon* Lynn Savoie* * MD at time of publication
Aplastic anemia: hypo-proliferation of bone marrow RBC precursors
Anemia of Chronic Disease
Splenomegaly
↑ RBC sequestration within enlarged spleen
Acute bleeding
Hemolysis (infection, autoimmune, RBC structural defects)
↓ RBC production
↑ RBC destruction/elimination
Normocytic Anemia:
[Hgb] <120g/L in females, <140g/L in males, with the RBC mean corpuscular volume (MCV) still within the normal range: 80-100 fL
RBCs that ultimately end up in the blood are still qualitatively normal/functional; there is a quantitative shortage of these RBCs in the blood relative to body needs
Spurious/False normocytic anemia:
Any fluid overload state (pregnancy, heart failure, kidney disease, etc.) can ↑ plasma volume which can dilute RBCs and cause apparent anemia, but the mean volume of each RBC is still normal
Normocytic Anemia
Heart needs to work faster to pump
sufficient oxygenated blood to tissues
↑ Heart rate
Reduced oxygen- carrying ability of blood
Patient feels oxygen- deprived, needs to inhale more oxygen as compensation
Dyspnea (shortness of breath) ↑ Respiratory rate (RR)
Not enough oxygen being delivered to body tissues, including brain
Fatigue
Reduced absolute number of RBCs means less RBCs to color the blood red
Pallor (especially conjunctival and palmar)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-Published July 27, 2019 on www.thecalgaryguide.com
Normocytic Anemia: Causes, Signs, and Symptoms
Authors: Katie Lin Yan Yu Reviewers: Andrew Brack Jessica Tjong Man-Chiu Poon* Lynn Savoie* * MD at time of publication
Aplastic anemia: hypo-proliferation of bone marrow RBC precursors
Anemia of Chronic Disease
Splenomegaly
↑ RBC sequestration within enlarged spleen
Acute bleeding
Hemolysis (infection, autoimmune, RBC structural defects)
↓ RBC production
↑ RBC destruction/elimination
Normocytic Anemia:
[Hgb] <120g/L in females, <140g/L in males, with the RBC mean corpuscular volume (MCV) still within the normal range: 80-100 fL
RBCs that ultimately end up in the blood are still qualitatively normal/functional; there is a quantitative shortage of these RBCs in the blood relative to body needs
Spurious/False normocytic anemia:
Any fluid overload state (pregnancy, heart failure, kidney disease, etc.) can ↑ plasma volume which can dilute RBCs and cause apparent anemia, but the mean volume of each RBC is still normal
Normocytic Anemia
Heart needs to work faster to pump
sufficient oxygenated blood to tissues
↑ Heart rate
Reduced oxygen- carrying ability of blood
Patient feels oxygen- deprived, needs to inhale more oxygen as compensation
Dyspnea (shortness of breath) ↑ Respiratory rate (RR)
Not enough oxygen being delivered to body tissues, including brain
Fatigue
Reduced absolute number of RBCs means less RBCs to color the blood red
Pallor (especially conjunctival and palmar)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-Published July 27, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Normocytic-Anemia-1.jpg)
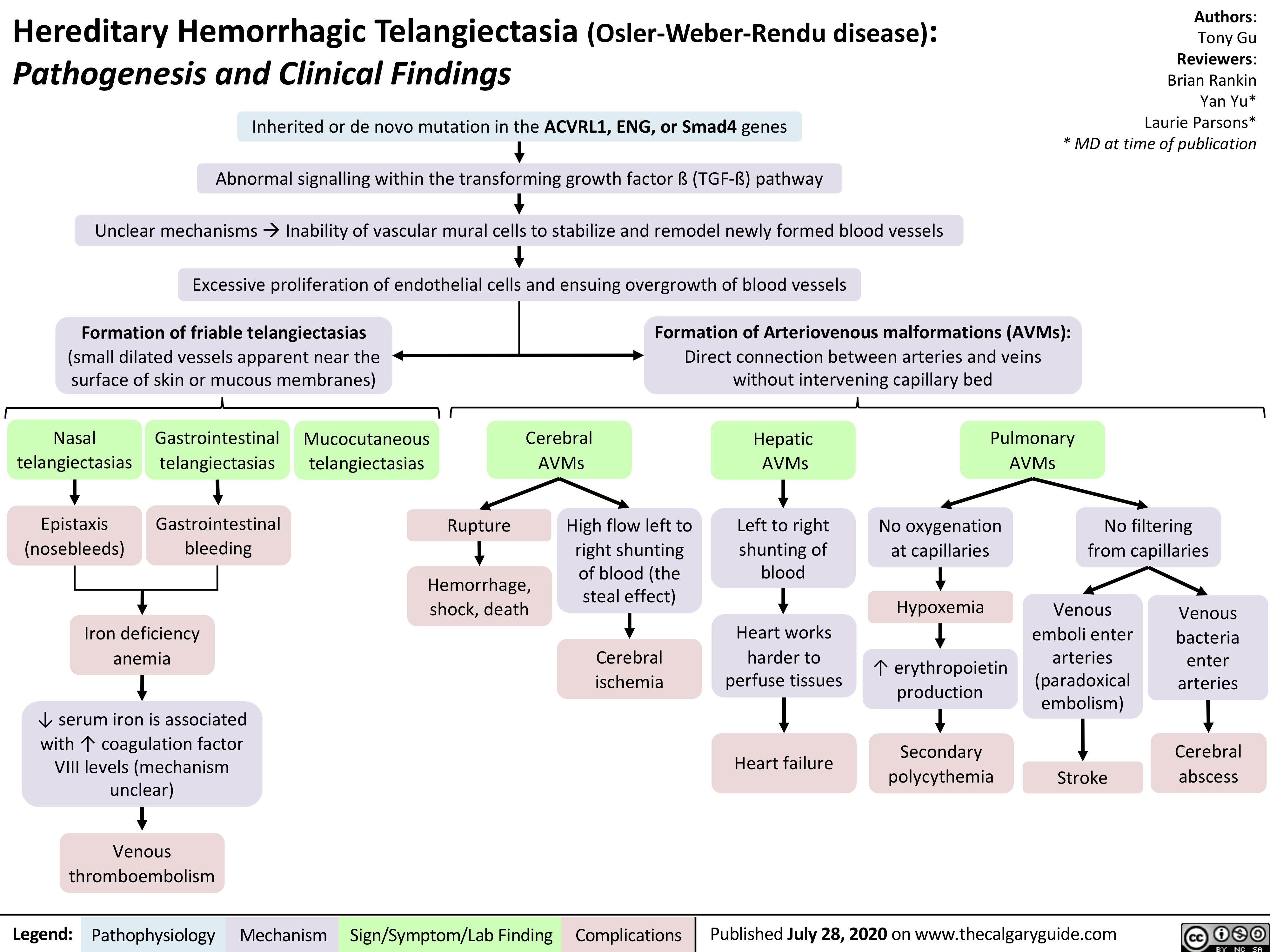
Hereditary Hemorrhagic Telangiectasia (Osler-Weber-Rendu disease)
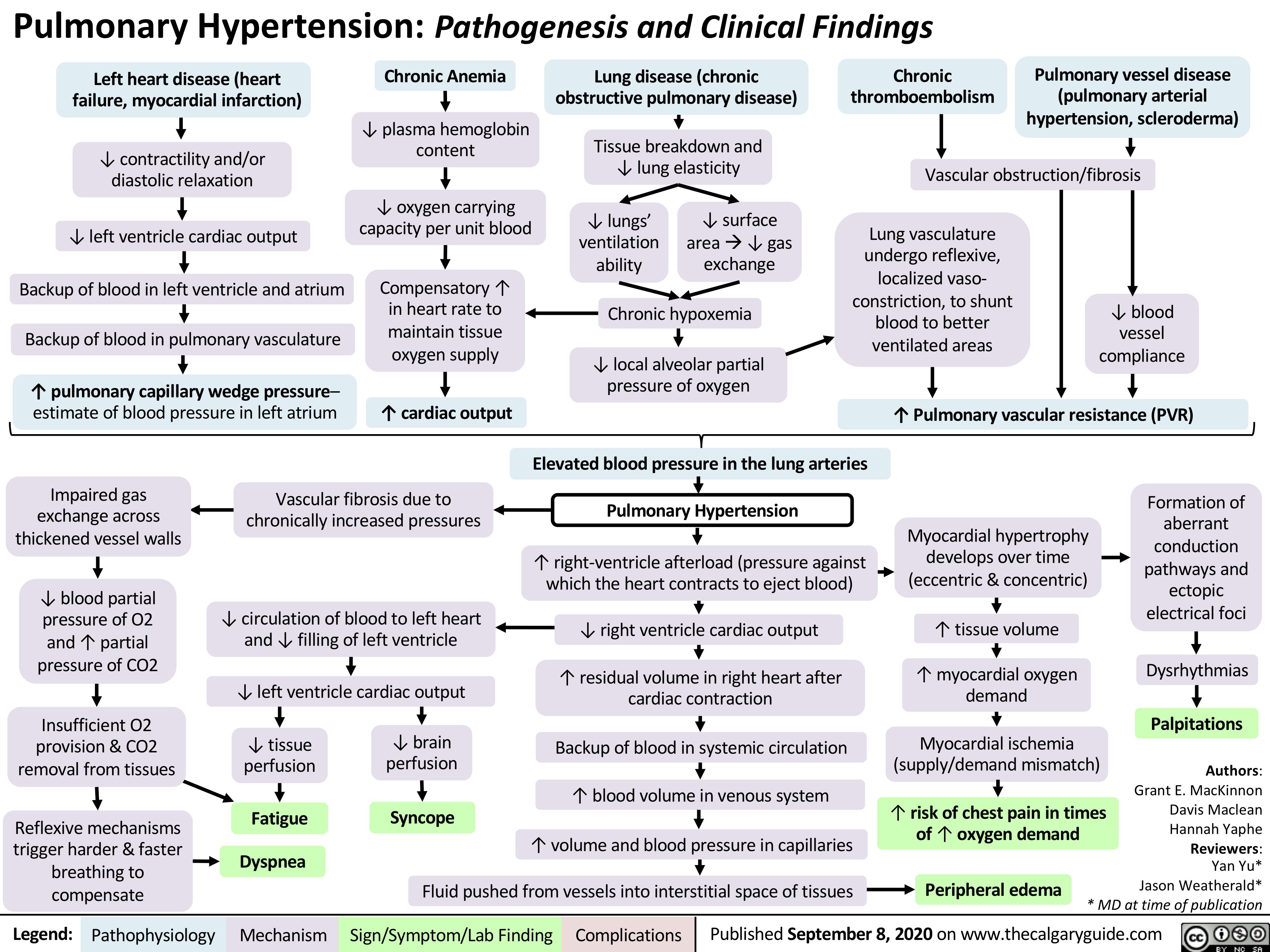
Pulmonary Hypertension
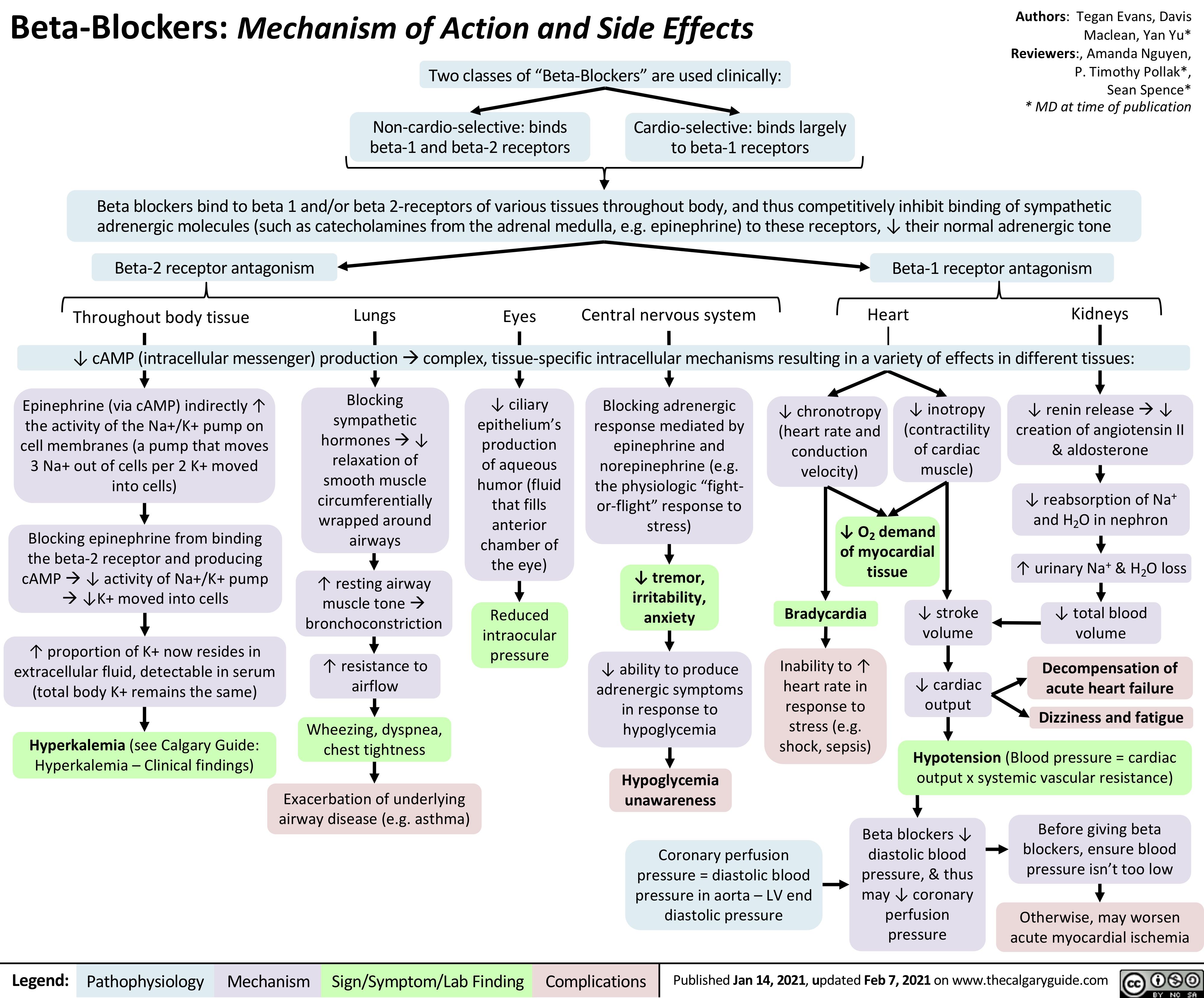
Beta-Blockers-Mechanism-of-Action-and-Side-Effects
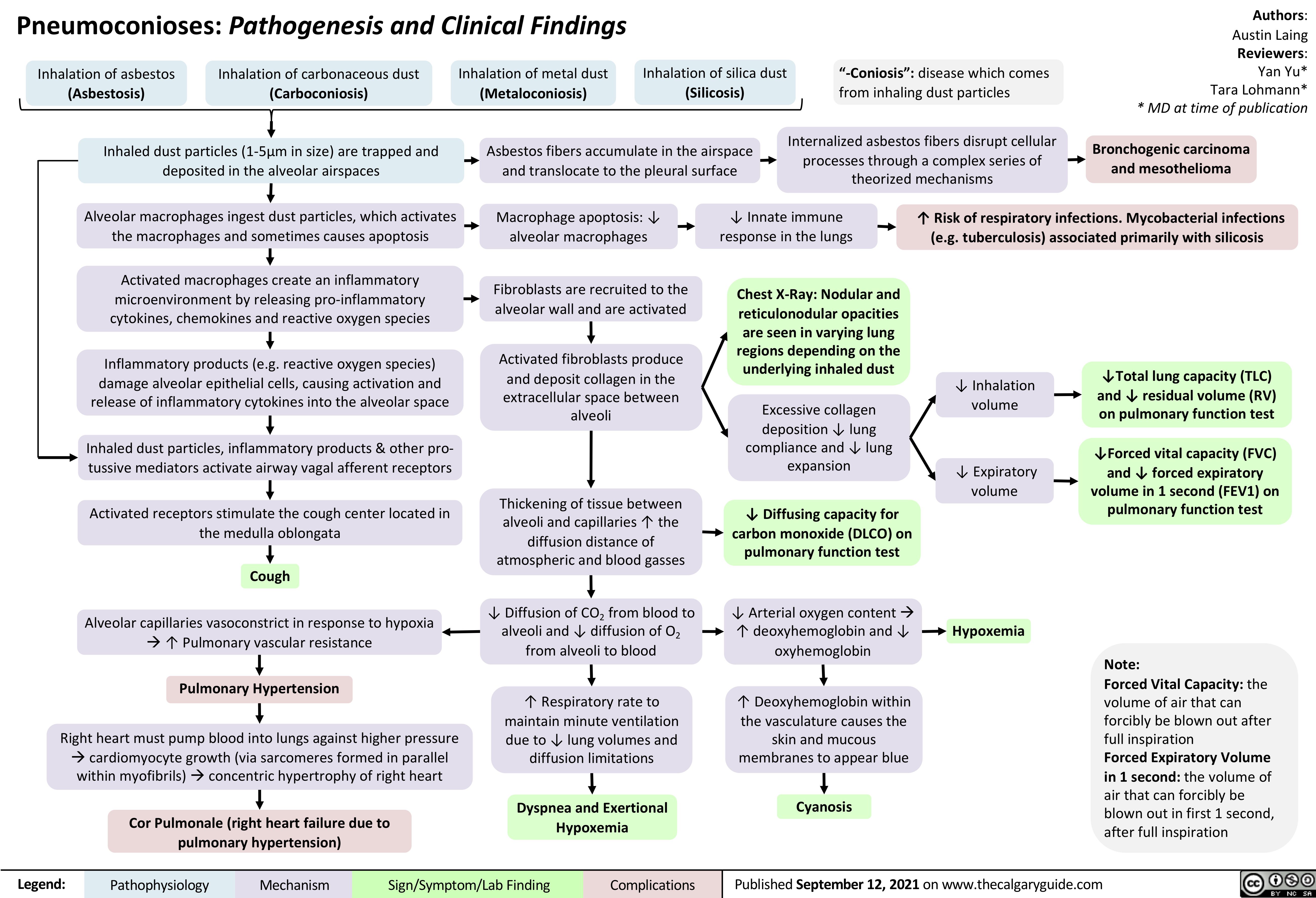
Pneumoconioses
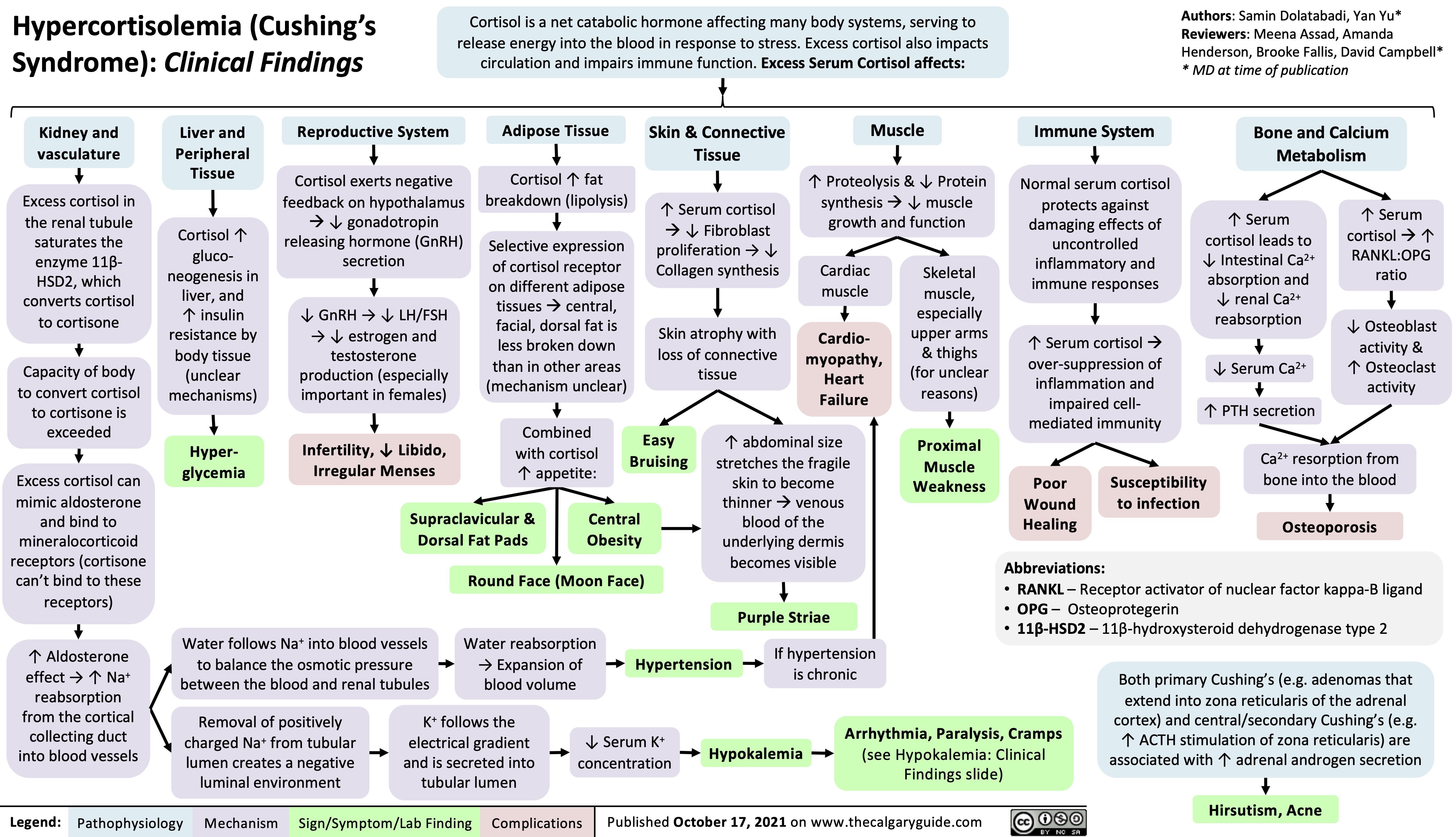
Hypercortisolemia
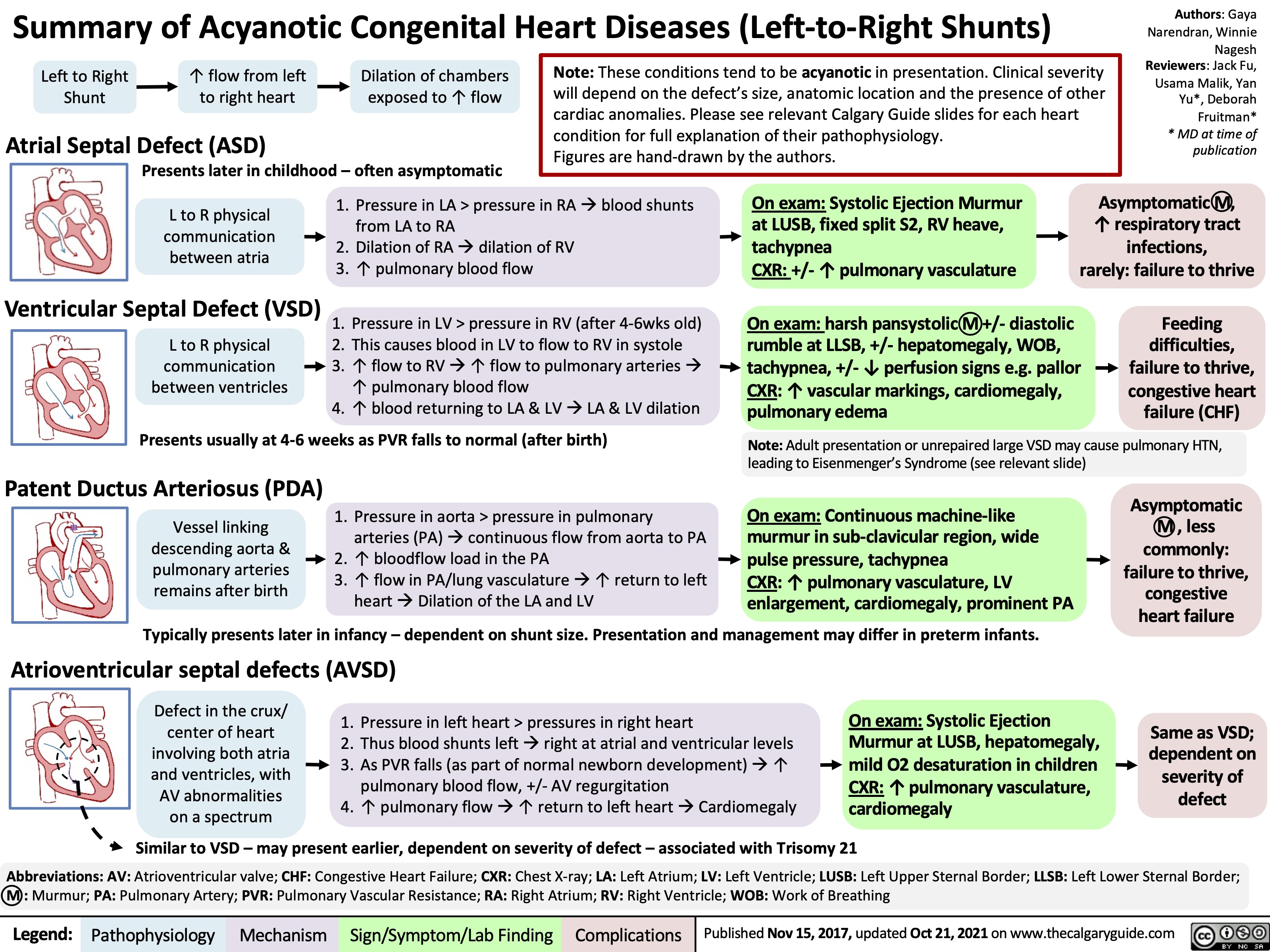
Summary of Acyanotic Congenital Heart Diseases
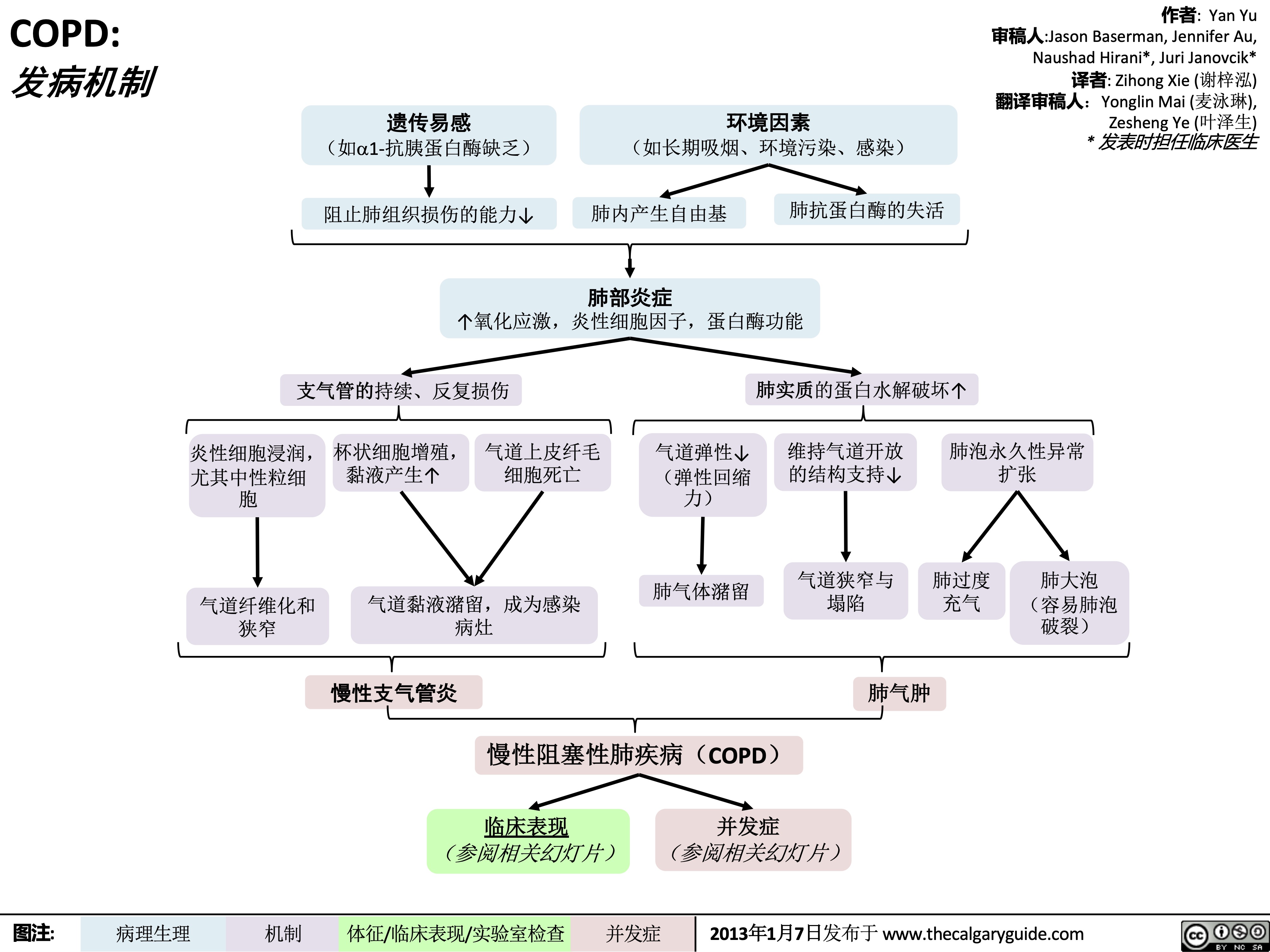
COPD-发病机制
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
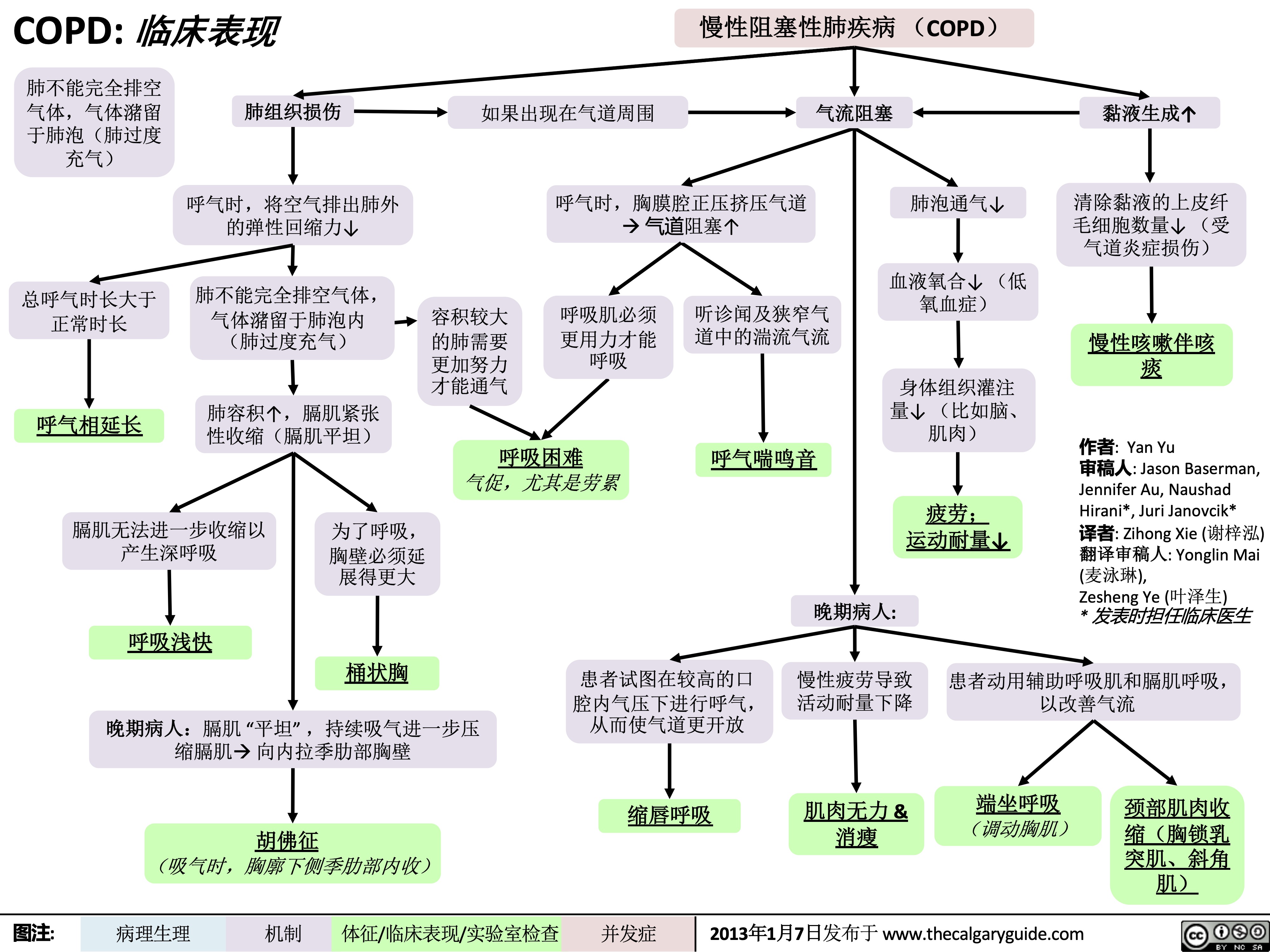
COPD-临床表现
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
chronic-hypertension-complications
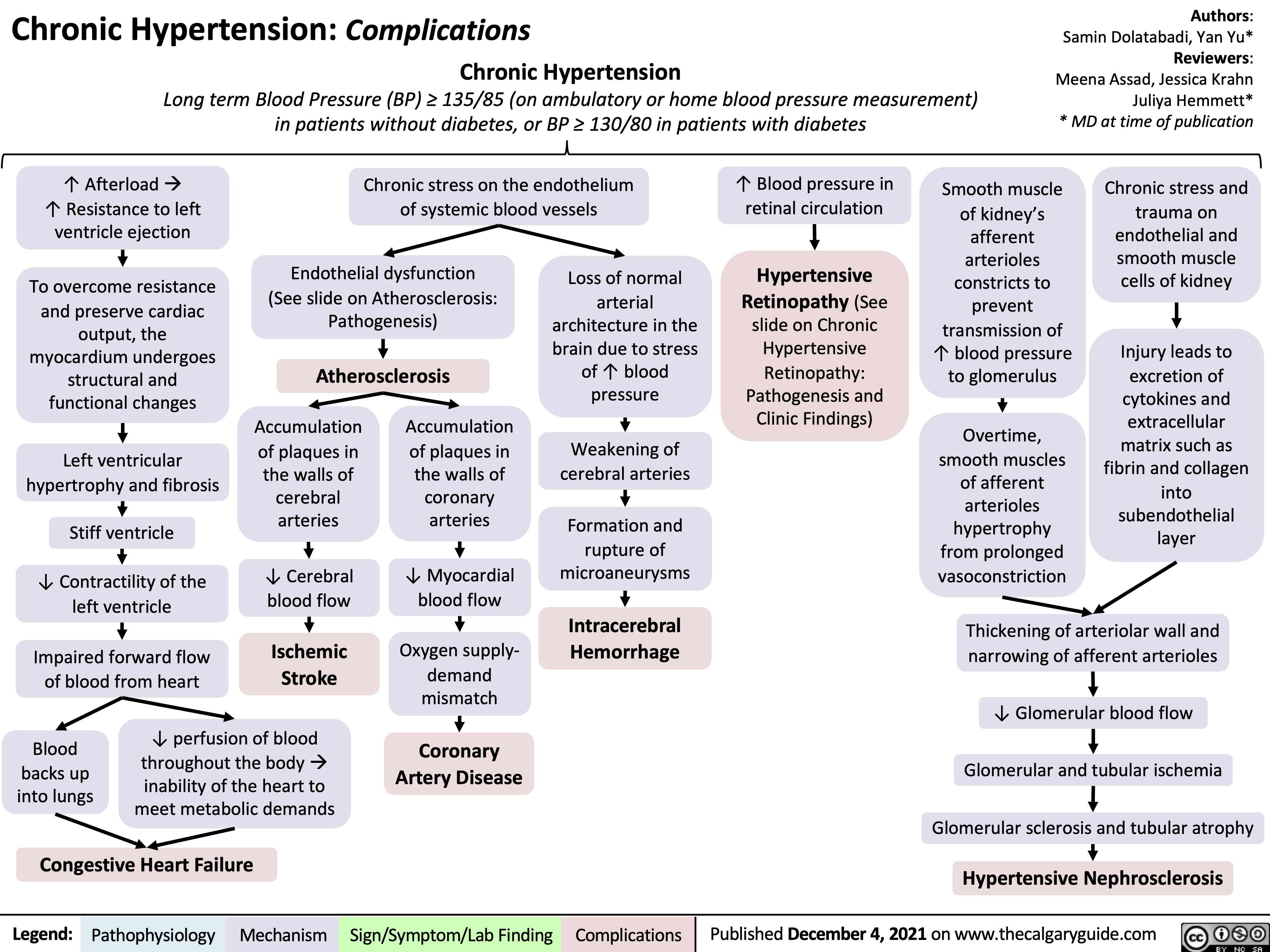
Renal Artery Stenosis
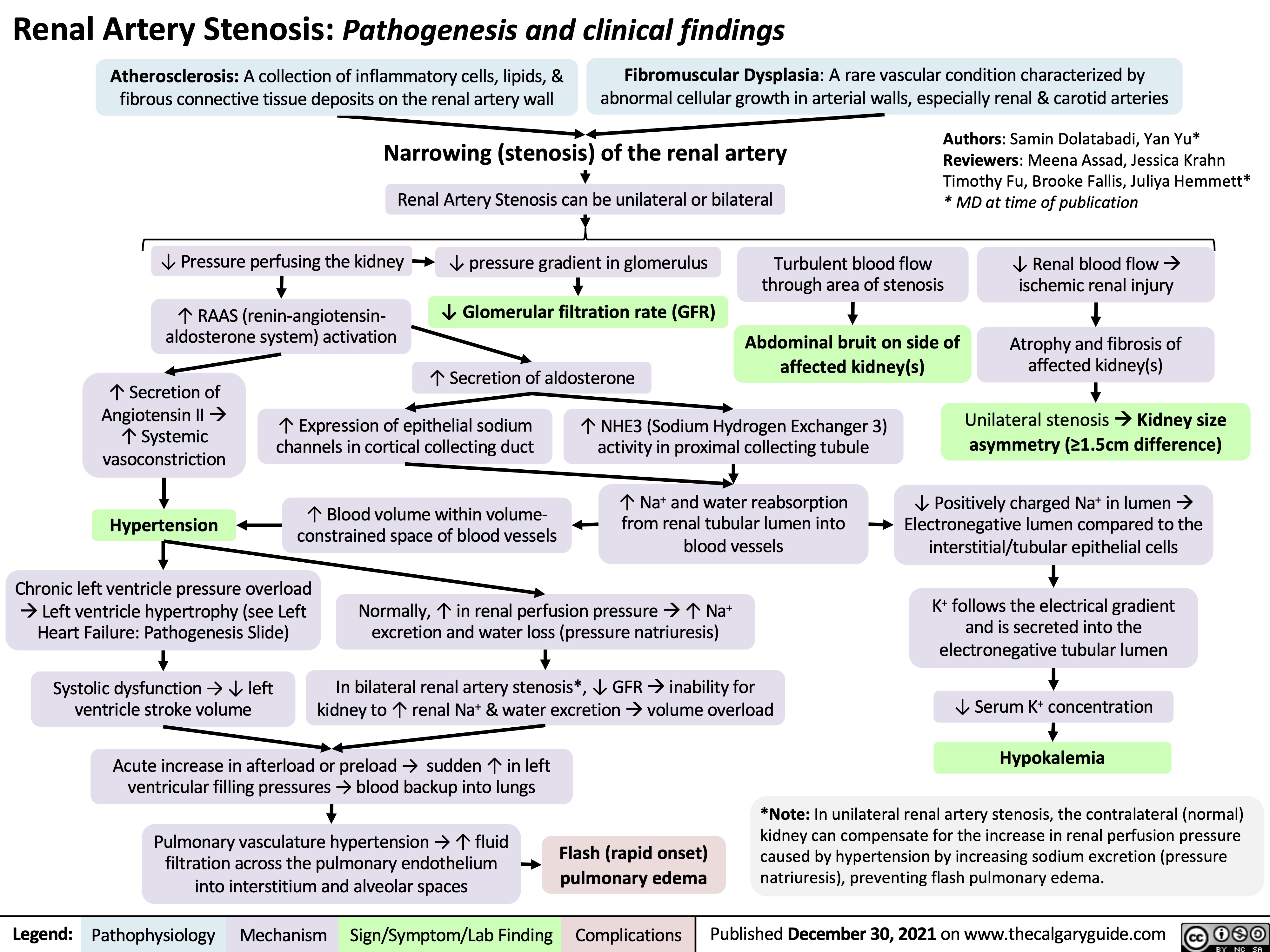
complications-of-pulmonary-embolism
![Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com
Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/Complications-of-Pulmonary-Embolism-2022.jpg)
obstructive-sleep-apnea-pathogenesis-and-clinical-findings
![Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新
Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/OSA-2021-1.jpg)
transudative-pleural-effusions-pathogenesis-and-lab-findings
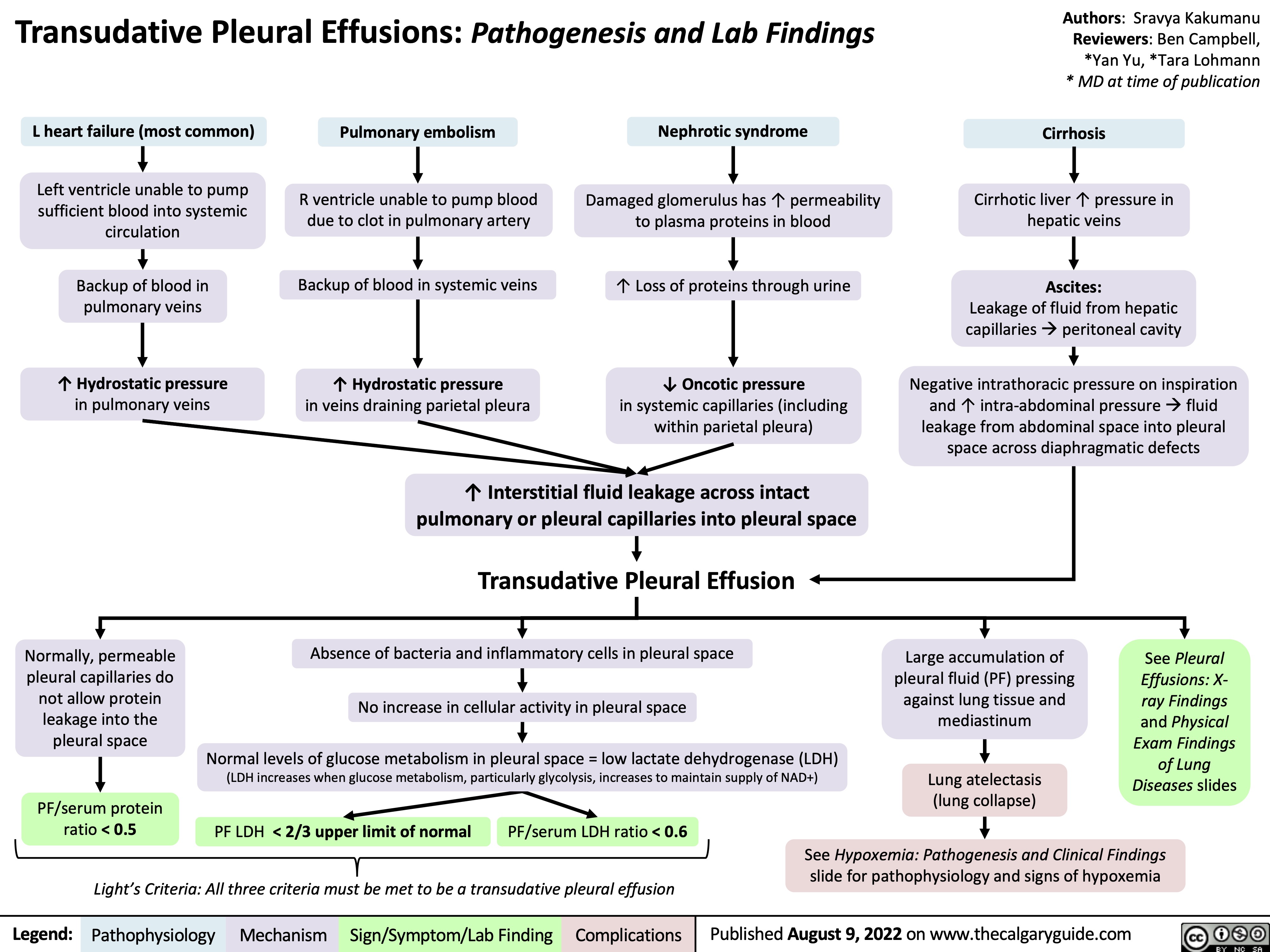
chest-exam-findings-of-lung-pleural-diseases
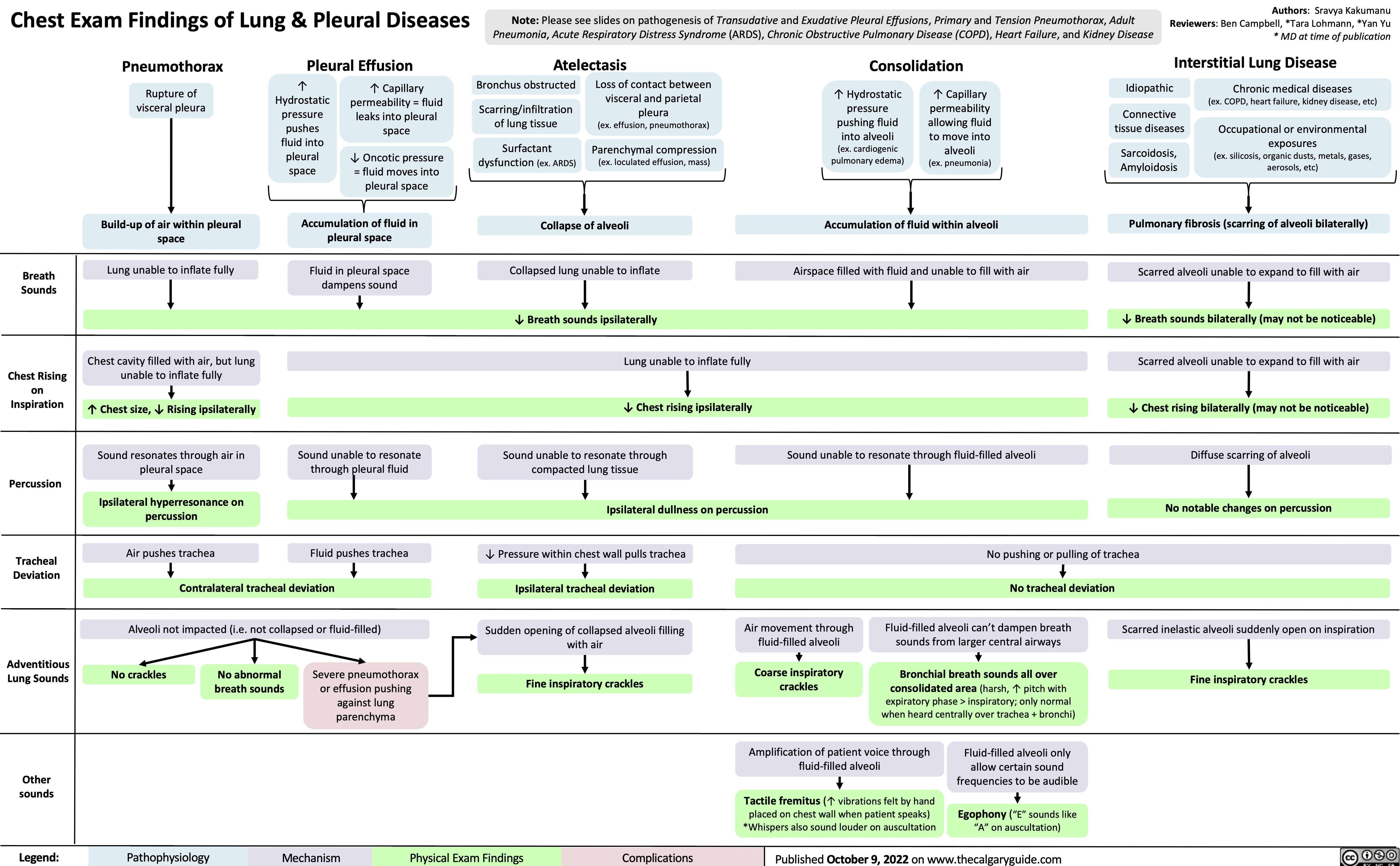
Coronary Artery Bypass Graft CABG Indications
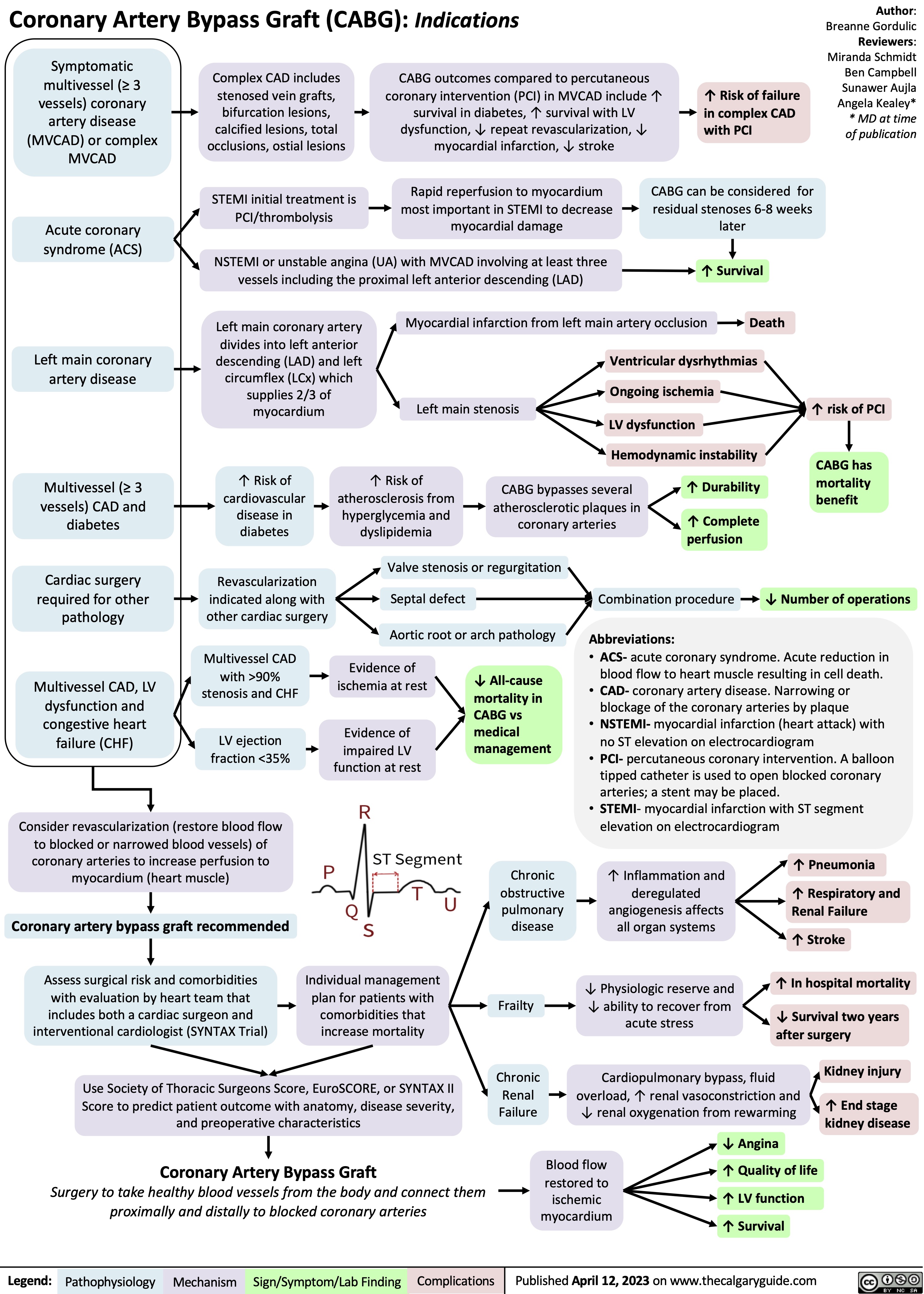
Mitral Regurgitation Pathogenesis and clinical findings
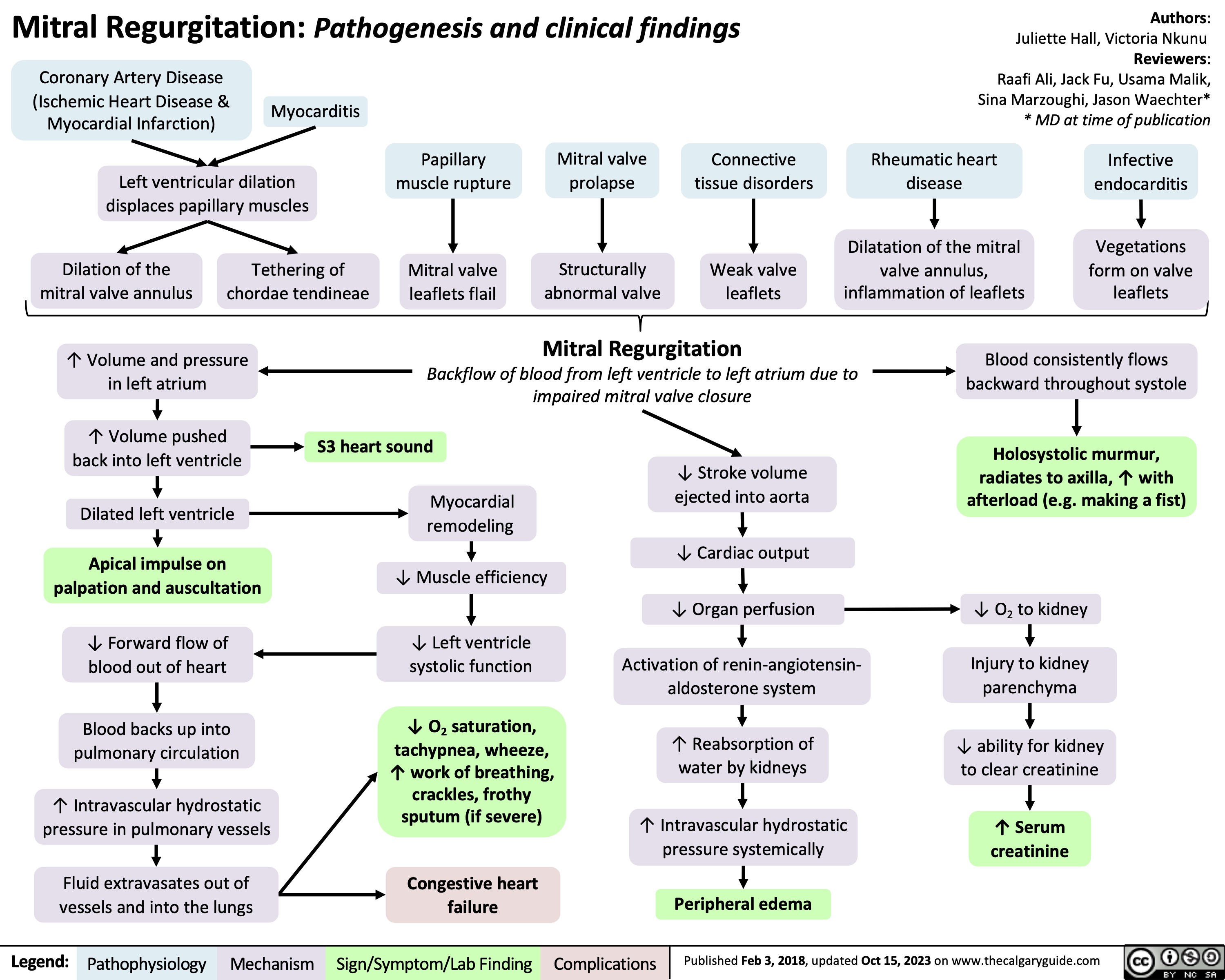
Death Cardiovascular Respiratory and Neurologic Mechanisms
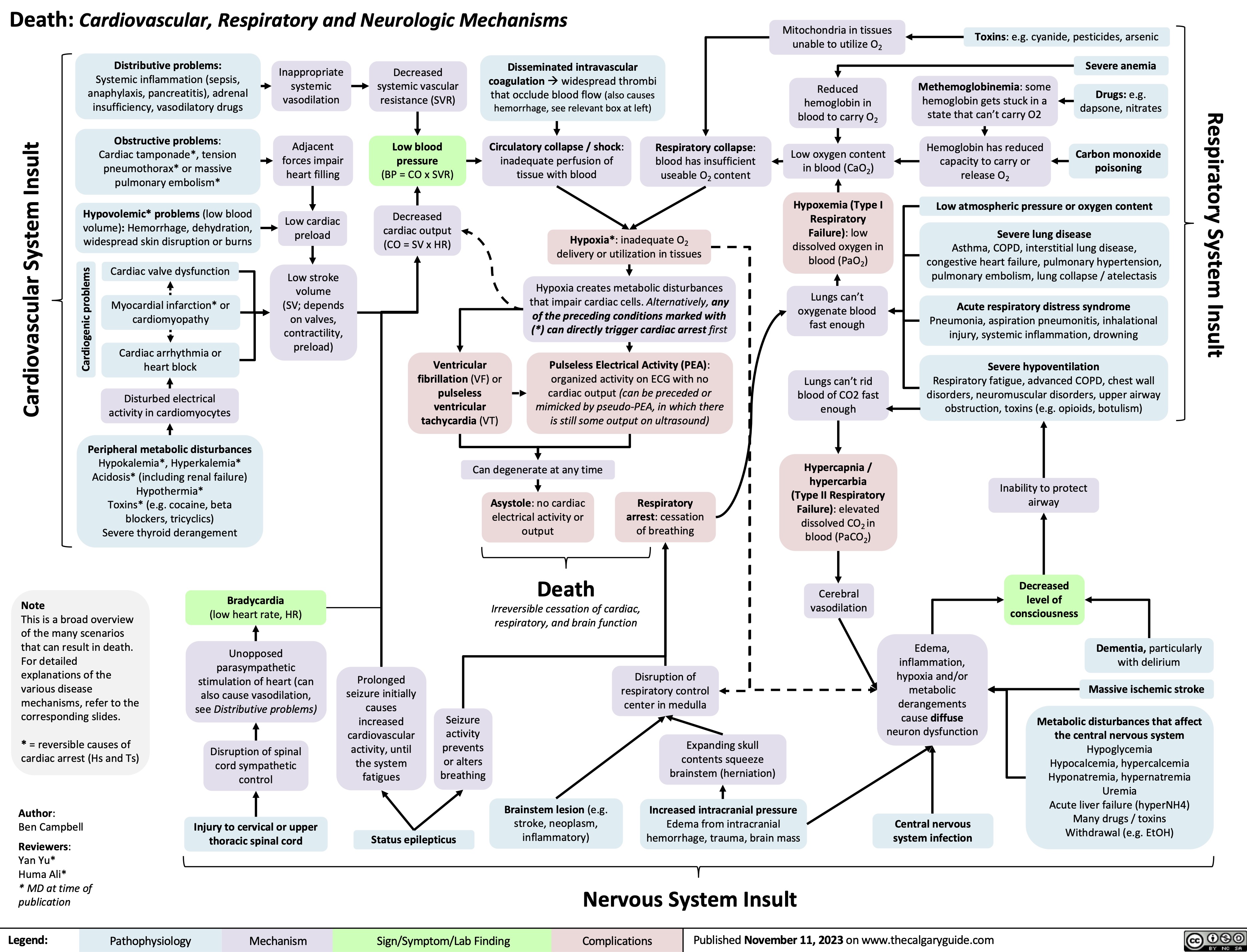
Eisenmenger Syndrome
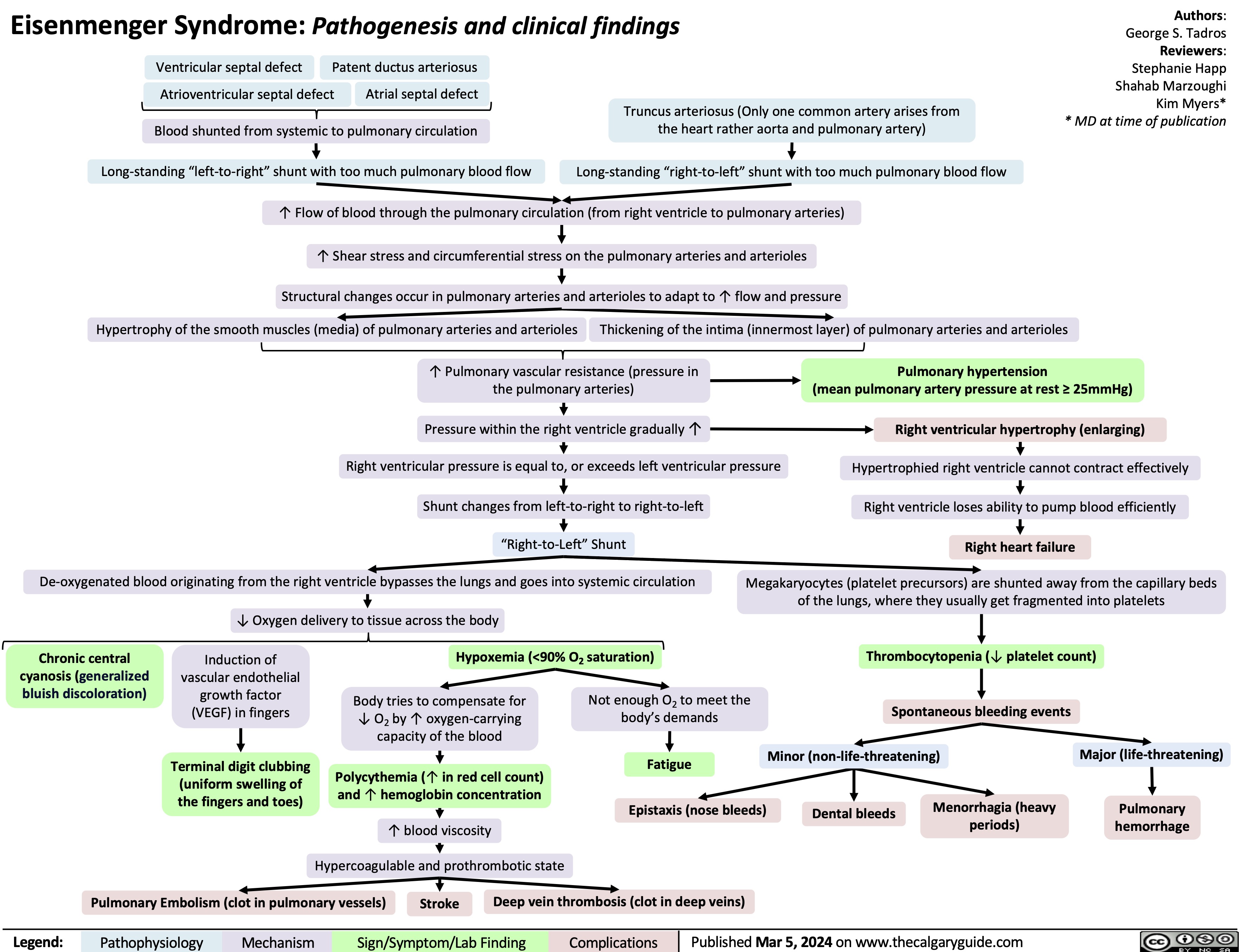
Atrial Septal Defect Pathogenesis and Clinical Findings
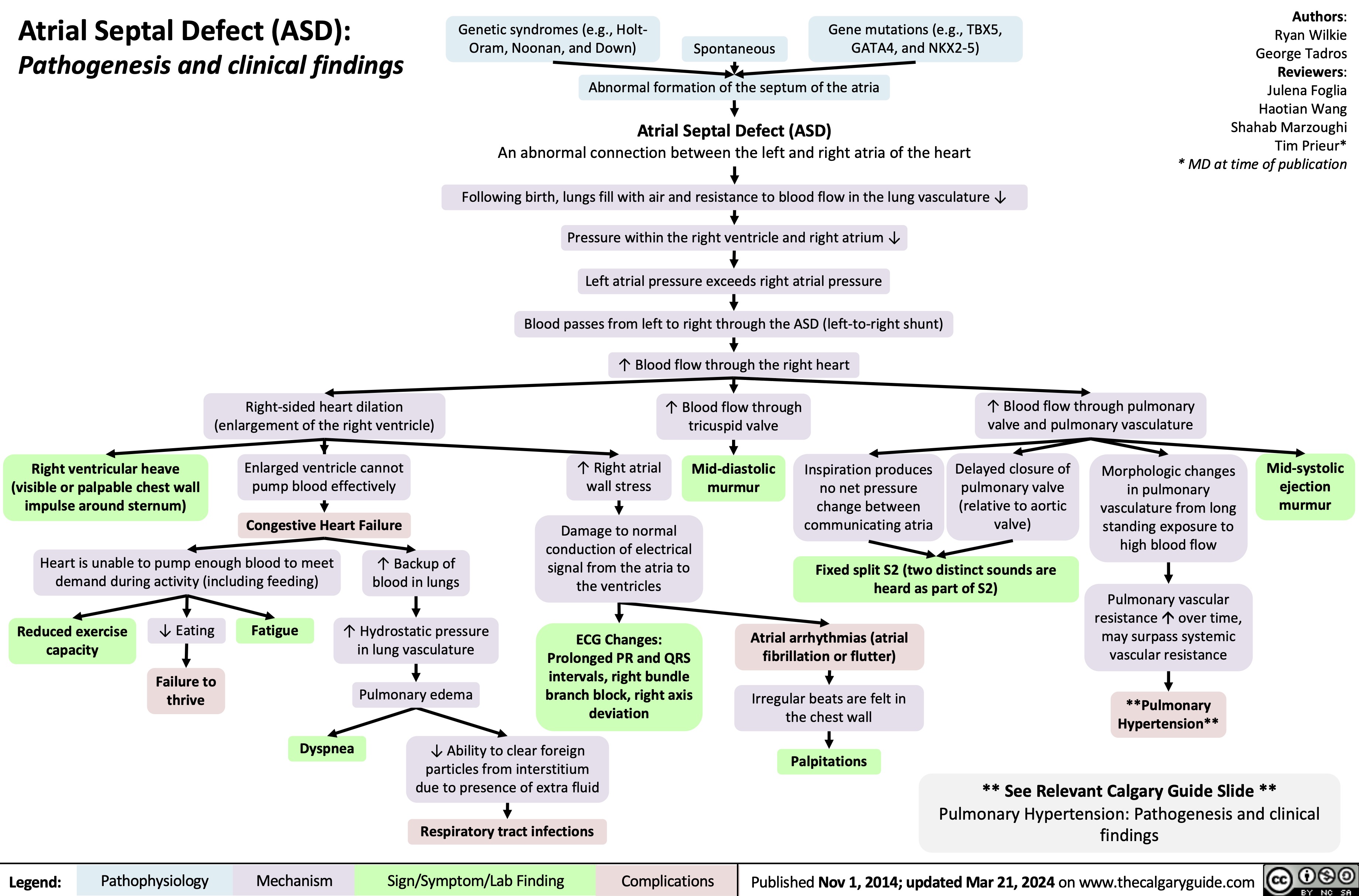
Gynecomastia
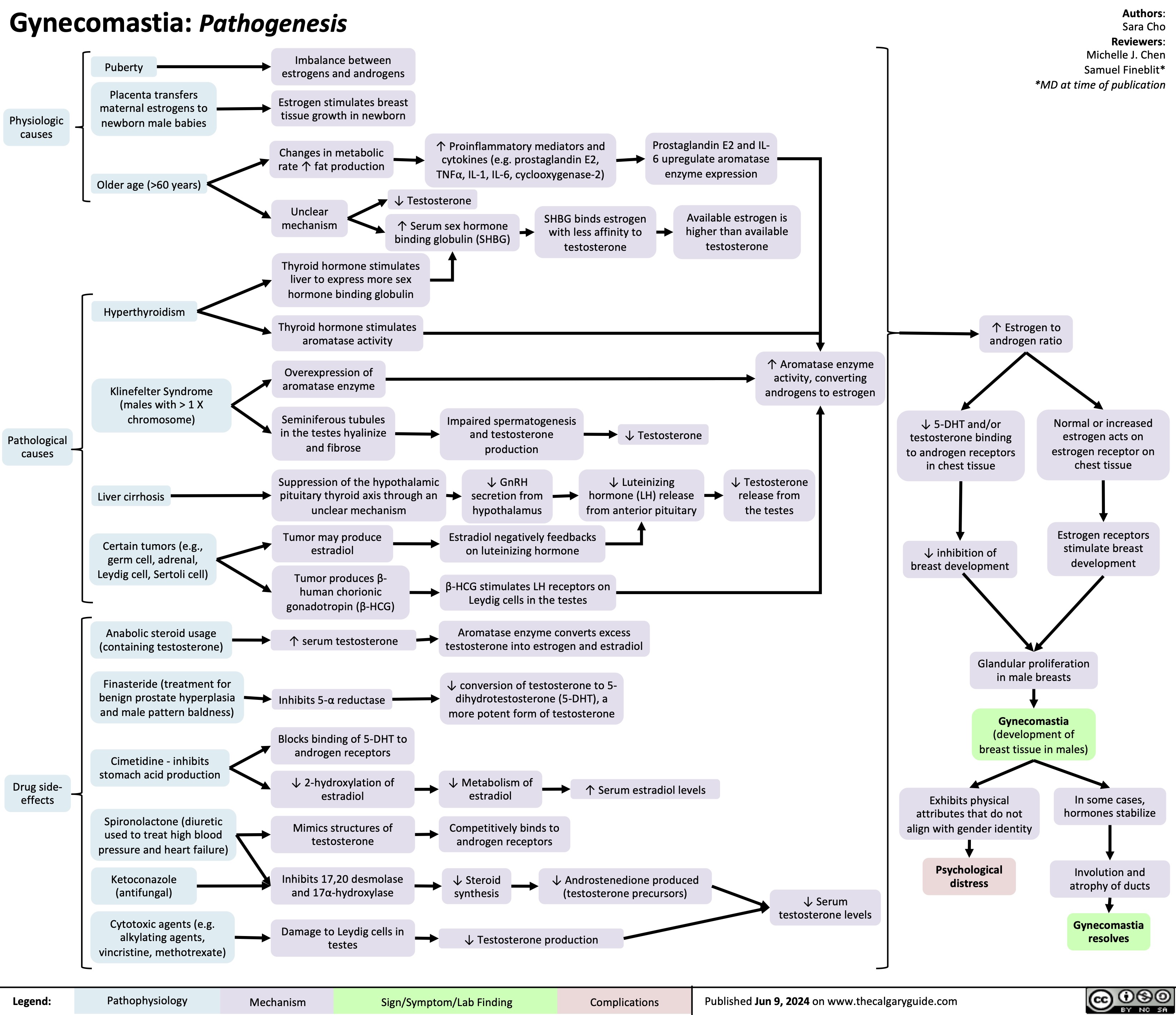
Underfill Edema Pathogenesis
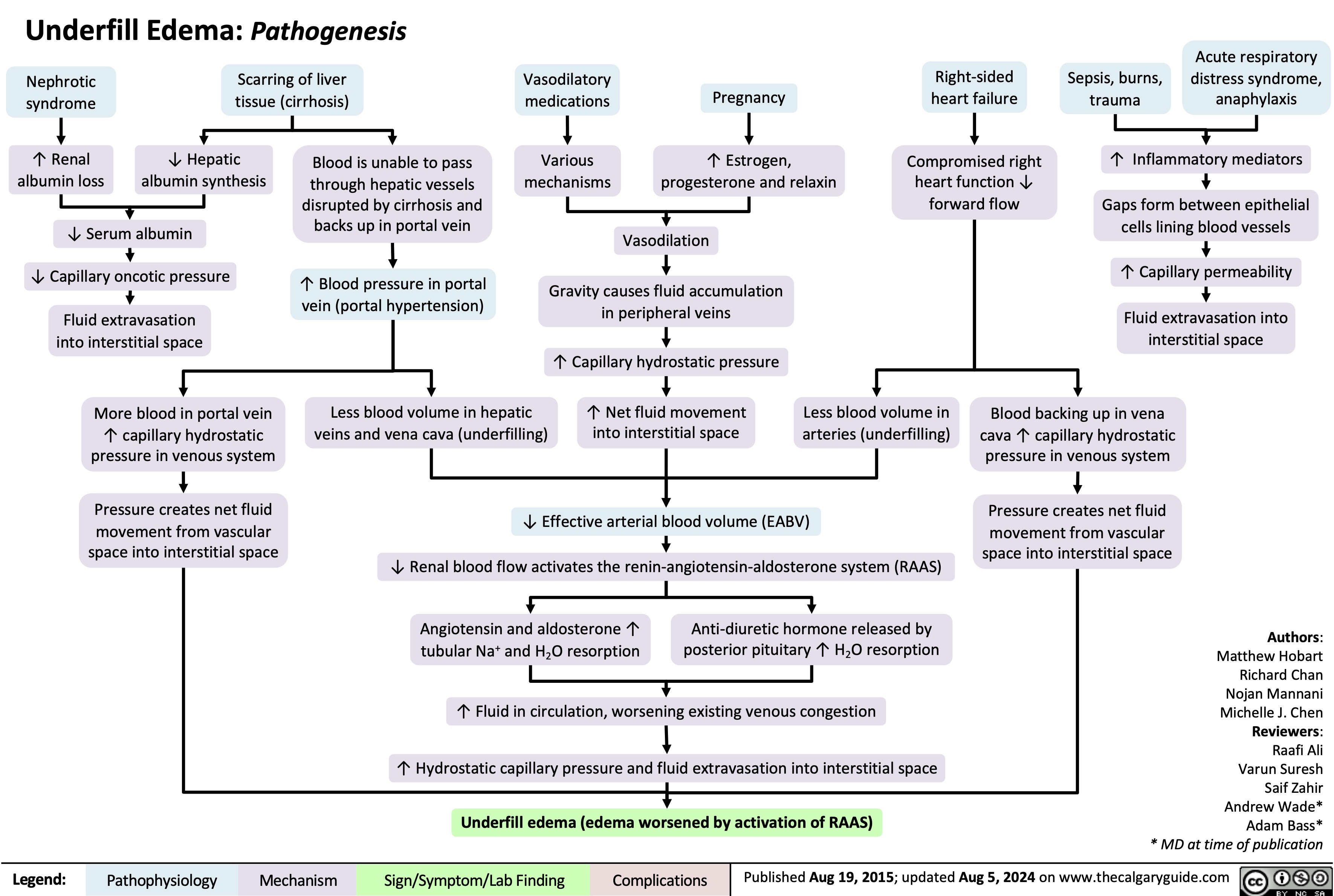
S3 Pathogenesis
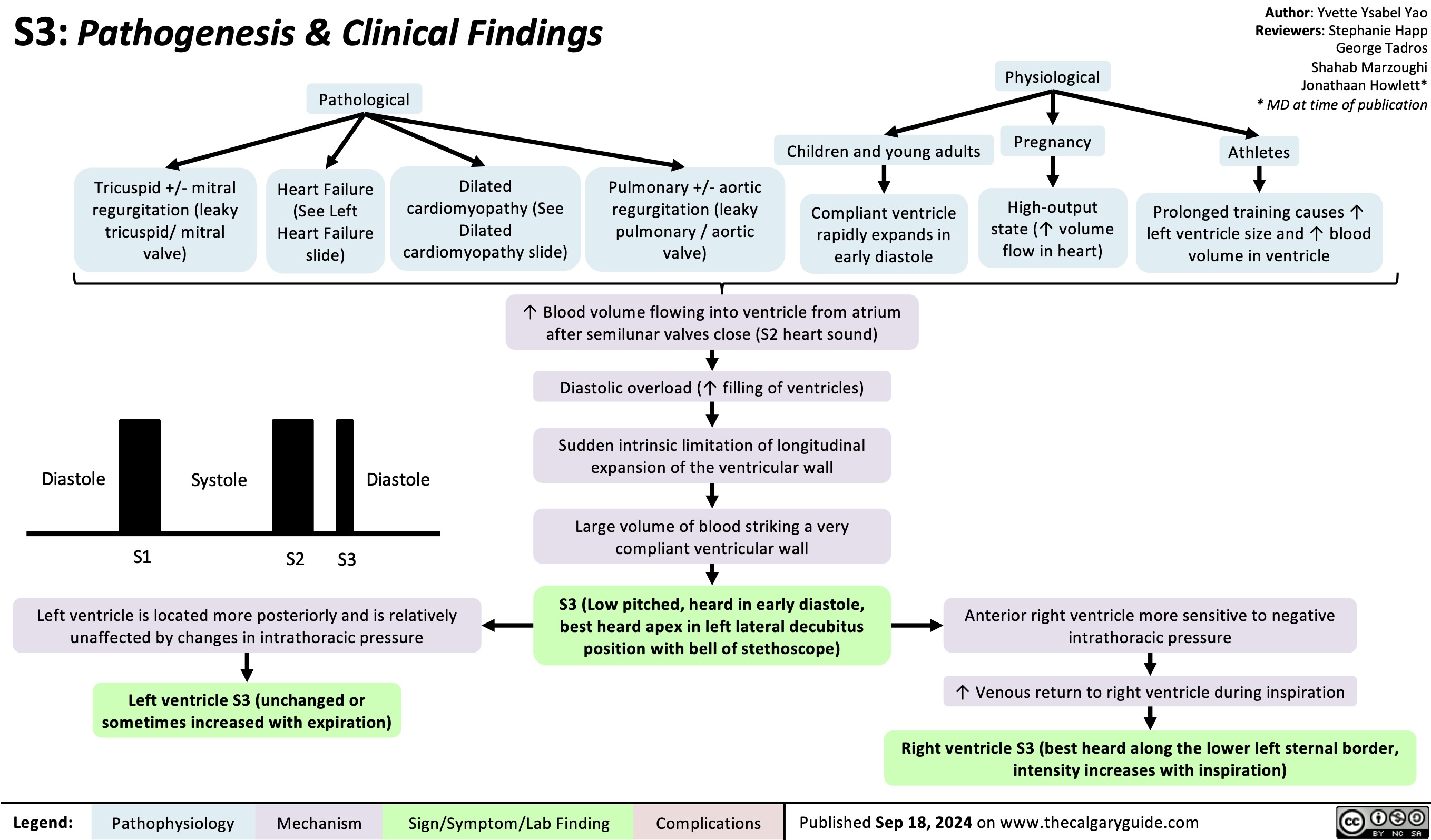
Hyperthyroidism in Pregnancy
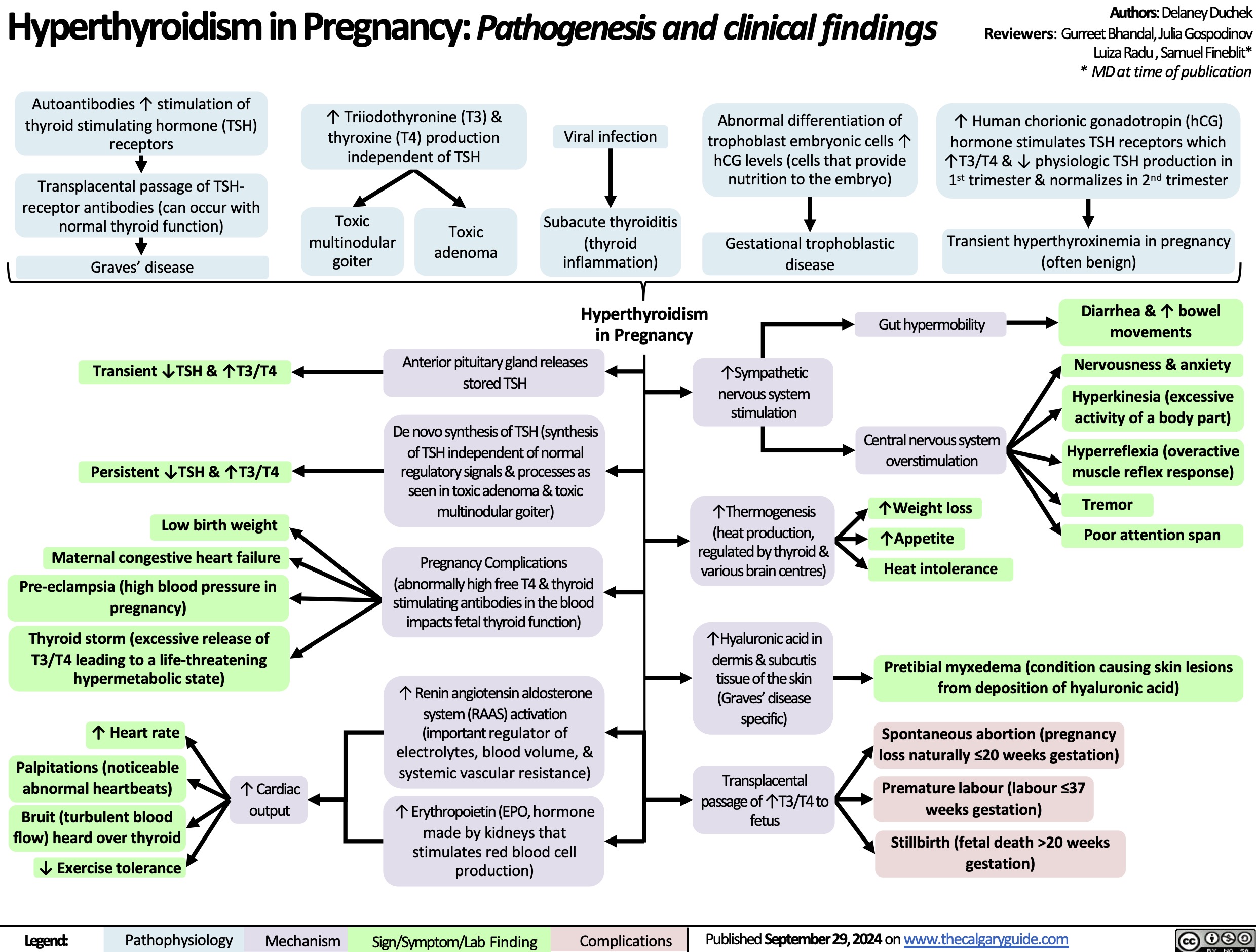
Persistent Truncus Arteriosus
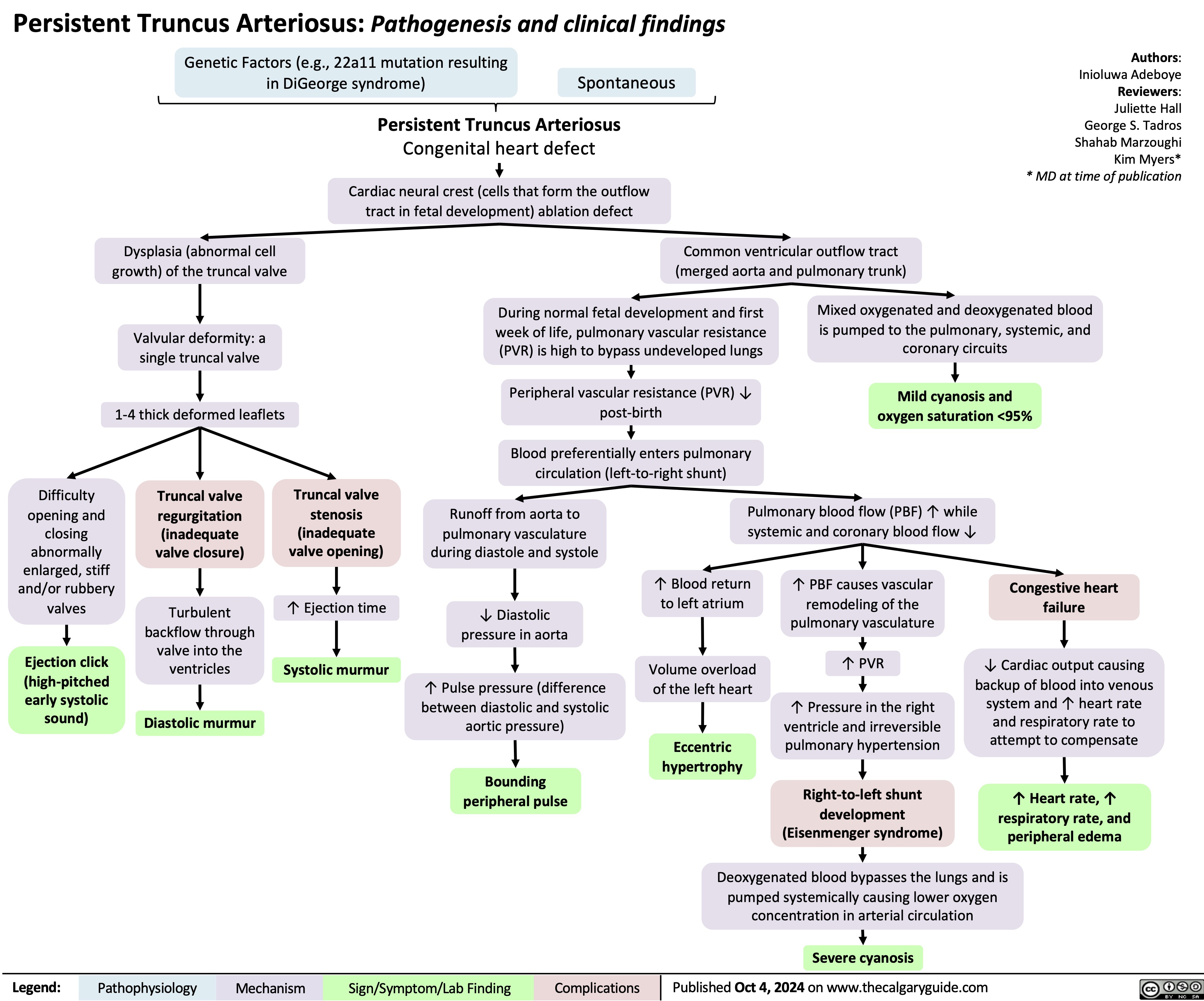
Calcium Channel Blockers
![Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com
Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/11/Calcium-Channel-Blockers.jpg)







![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)


![Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hyperkalemia-Physiology-.jpg)
![Torsades de Pointes (TdP): Pathogenesis and Clinical Findings
Drugs (e.g. Class 1A [quinidine], Class III [sotalol,
amiodarone], TCAs, erythromycin, quinolones, anti-histamines)
Sinus bradycardia, AV block
Metabolic abnormality (hypo K+/Ca2+/Mg2+)
Primary heart disease: ischemic, congestive heart failure, cardiomyopathy
Acquired long QT syndrome
Congenital long QT syndrome
↓ repolarizing current/ depolarizing current in cardiomyocytes
Mutated cardiac ion channels
Author: Nicola Adderley Reviewers: Luke Gagnon Emily Ryznar *Saman Rezazadeh *George Veenhuyzen MD at time of publication*
↓ repolarizing current in cardiomyocytes
QTc interval
Prolonged ventricular action potential duration
Early after depolarization (EAD) triggering PVC
Torsades de Pointes
Illustrated changes to action potential:
Normal cardiac action potential
EAD
Abbreviations: TCAs: tricyclic antidepressants AV: atrioventricular QTc: QT interval, corrected for heart rate
PVC: premature ventricular contraction
VF: ventricular fibrillation
SR: sinus rhythm
Polymorphic ventricular tachycardia initiated by PVC in the setting of QT interval prolongation and maintained by functional re-entry
Non-sustained TdP
Asymptomatic, palpitations, syncope (length-dependent)
Illustrated changes to ECG Strip:
OR
Sinus rhythm restored
Degeneration to VF
Sudden cardiac death
Prolonged repolarization
Triggered beat (PVC)
A
BCDE
A. Prolonged QT interval B. PVC
C. PVC triggers TdP
D. Non-sustained TdP
E. Return to SR
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 10, 2019 on www.thecalgaryguide.com
Torsades de Pointes (TdP): Pathogenesis and Clinical Findings
Drugs (e.g. Class 1A [quinidine], Class III [sotalol,
amiodarone], TCAs, erythromycin, quinolones, anti-histamines)
Sinus bradycardia, AV block
Metabolic abnormality (hypo K+/Ca2+/Mg2+)
Primary heart disease: ischemic, congestive heart failure, cardiomyopathy
Acquired long QT syndrome
Congenital long QT syndrome
↓ repolarizing current/ depolarizing current in cardiomyocytes
Mutated cardiac ion channels
Author: Nicola Adderley Reviewers: Luke Gagnon Emily Ryznar *Saman Rezazadeh *George Veenhuyzen MD at time of publication*
↓ repolarizing current in cardiomyocytes
QTc interval
Prolonged ventricular action potential duration
Early after depolarization (EAD) triggering PVC
Torsades de Pointes
Illustrated changes to action potential:
Normal cardiac action potential
EAD
Abbreviations: TCAs: tricyclic antidepressants AV: atrioventricular QTc: QT interval, corrected for heart rate
PVC: premature ventricular contraction
VF: ventricular fibrillation
SR: sinus rhythm
Polymorphic ventricular tachycardia initiated by PVC in the setting of QT interval prolongation and maintained by functional re-entry
Non-sustained TdP
Asymptomatic, palpitations, syncope (length-dependent)
Illustrated changes to ECG Strip:
OR
Sinus rhythm restored
Degeneration to VF
Sudden cardiac death
Prolonged repolarization
Triggered beat (PVC)
A
BCDE
A. Prolonged QT interval B. PVC
C. PVC triggers TdP
D. Non-sustained TdP
E. Return to SR
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 10, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Torsades-de-Pointes-TdP-Pathogenesis-and-Clinical-Findings-.jpg)

![Normocytic Anemia: Causes, Signs, and Symptoms
Authors: Katie Lin Yan Yu Reviewers: Andrew Brack Jessica Tjong Man-Chiu Poon* Lynn Savoie* * MD at time of publication
Aplastic anemia: hypo-proliferation of bone marrow RBC precursors
Anemia of Chronic Disease
Splenomegaly
↑ RBC sequestration within enlarged spleen
Acute bleeding
Hemolysis (infection, autoimmune, RBC structural defects)
↓ RBC production
↑ RBC destruction/elimination
Normocytic Anemia:
[Hgb] <120g/L in females, <140g/L in males, with the RBC mean corpuscular volume (MCV) still within the normal range: 80-100 fL
RBCs that ultimately end up in the blood are still qualitatively normal/functional; there is a quantitative shortage of these RBCs in the blood relative to body needs
Spurious/False normocytic anemia:
Any fluid overload state (pregnancy, heart failure, kidney disease, etc.) can ↑ plasma volume which can dilute RBCs and cause apparent anemia, but the mean volume of each RBC is still normal
Normocytic Anemia
Heart needs to work faster to pump
sufficient oxygenated blood to tissues
↑ Heart rate
Reduced oxygen- carrying ability of blood
Patient feels oxygen- deprived, needs to inhale more oxygen as compensation
Dyspnea (shortness of breath) ↑ Respiratory rate (RR)
Not enough oxygen being delivered to body tissues, including brain
Fatigue
Reduced absolute number of RBCs means less RBCs to color the blood red
Pallor (especially conjunctival and palmar)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-Published July 27, 2019 on www.thecalgaryguide.com
Normocytic Anemia: Causes, Signs, and Symptoms
Authors: Katie Lin Yan Yu Reviewers: Andrew Brack Jessica Tjong Man-Chiu Poon* Lynn Savoie* * MD at time of publication
Aplastic anemia: hypo-proliferation of bone marrow RBC precursors
Anemia of Chronic Disease
Splenomegaly
↑ RBC sequestration within enlarged spleen
Acute bleeding
Hemolysis (infection, autoimmune, RBC structural defects)
↓ RBC production
↑ RBC destruction/elimination
Normocytic Anemia:
[Hgb] <120g/L in females, <140g/L in males, with the RBC mean corpuscular volume (MCV) still within the normal range: 80-100 fL
RBCs that ultimately end up in the blood are still qualitatively normal/functional; there is a quantitative shortage of these RBCs in the blood relative to body needs
Spurious/False normocytic anemia:
Any fluid overload state (pregnancy, heart failure, kidney disease, etc.) can ↑ plasma volume which can dilute RBCs and cause apparent anemia, but the mean volume of each RBC is still normal
Normocytic Anemia
Heart needs to work faster to pump
sufficient oxygenated blood to tissues
↑ Heart rate
Reduced oxygen- carrying ability of blood
Patient feels oxygen- deprived, needs to inhale more oxygen as compensation
Dyspnea (shortness of breath) ↑ Respiratory rate (RR)
Not enough oxygen being delivered to body tissues, including brain
Fatigue
Reduced absolute number of RBCs means less RBCs to color the blood red
Pallor (especially conjunctival and palmar)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-Published July 27, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Normocytic-Anemia-1.jpg)






 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />


![Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com
Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/Complications-of-Pulmonary-Embolism-2022.jpg)
![Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新
Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/OSA-2021-1.jpg)












![Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com
Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/11/Calcium-Channel-Blockers.jpg)
